Pan02 – pancreatic ductal adenocarcinoma model characterized for immuno-oncology applications
AUTHOR:
Dylan Daniel,博士,科学发展总监
日期:
2017年7月
胰腺导管腺癌(PDAC)是最常见的胰腺癌,约占所有病例的95%。2017年,美国将有约50987人被诊断为PDAC,约40936名患者死亡,使PDAC成为最致命的癌症之一。联合抗代谢物和抗有丝分裂紫杉烷为基础的化疗是美国的标准治疗方案,但这些治疗方案只能提高总生存数周。对于治疗胰腺癌的新的治疗方法有一个关键的未满足的医学需求。
Because so many novel approaches to treat pancreatic cancer have met with disappointing clinical outcomes, many drug discovery scientists have turned to immunotherapy in pancreatic cancer based on the recent successes of harnessing the immune system to treat other cancers. Although there are a limited number of syngeneic mouse pancreatic cancer cell lines, Covance has characterized the Pan02 PDAC model for immuno-oncology applications. Pan02 was derived from C57BL/6 mice given orthotopic 3-methyl-cholanthrene and is refractory to many standard chemotherapeutic agents.1Pan02在细胞中含有功能缺失突变SMAD4型在大约30%的人类胰腺癌中功能类似于失活突变的基因。2
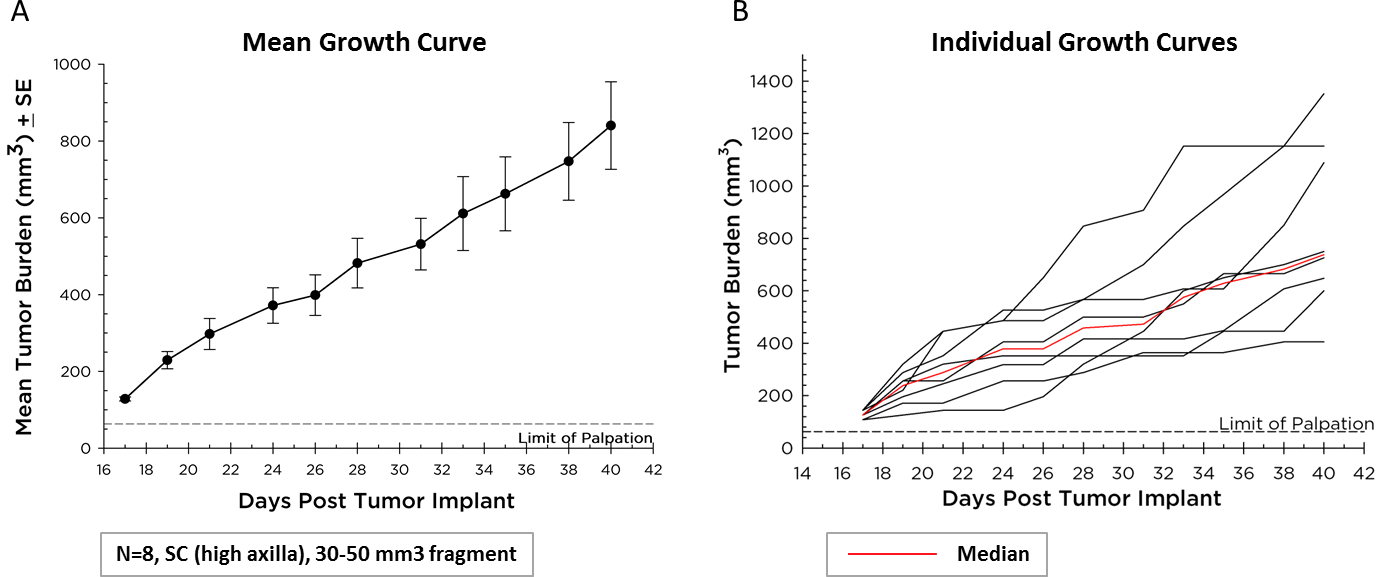
平均和个体生长曲线
Covance maintains Pan02 as a transplantable fragment model, and its mean growth kinetics (Figure 1A) and individual animal growth curves (Figure 1B) are illustrated. Pan02 has a mean tumor doubling time of six days which is slower than most syngeneic mouse cell line tumors. This slower growth may make it more tractable for immunotherapy since there is time for therapeutics to modify the immune system and elicit anti-tumor activity prior to tumors reaching euthanasia criteria.
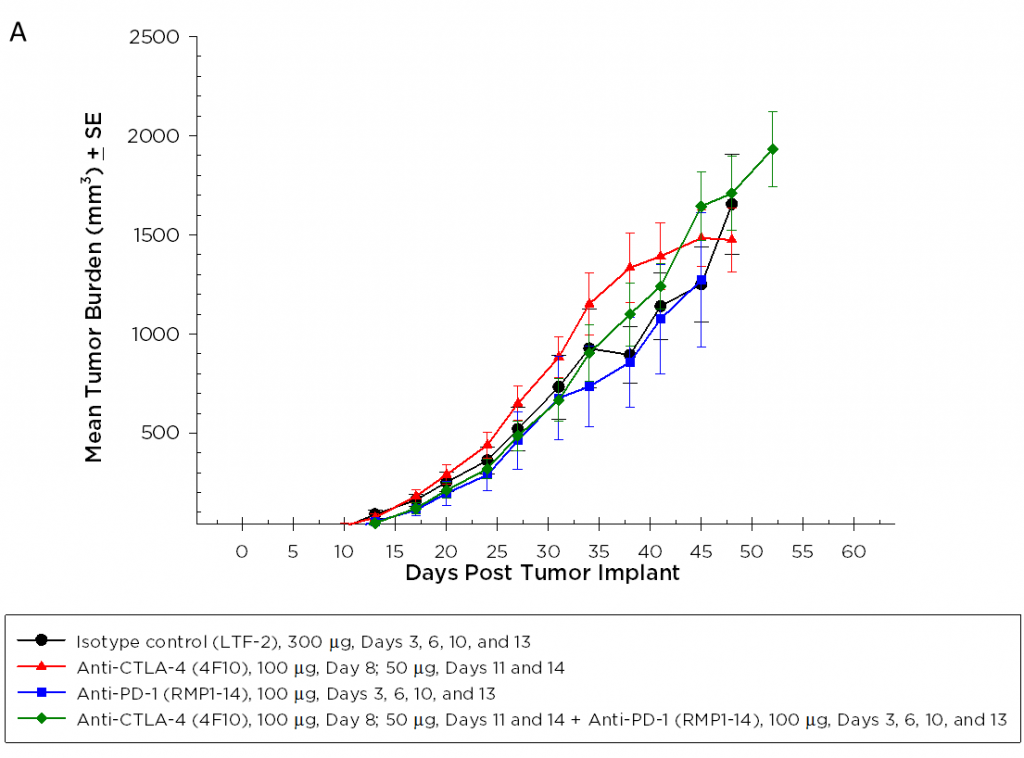
Anti-PD-1 and Anti-CTLA-4 Efficacy and Survival Rates
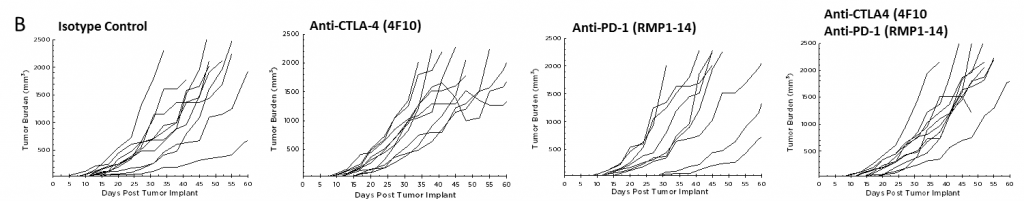
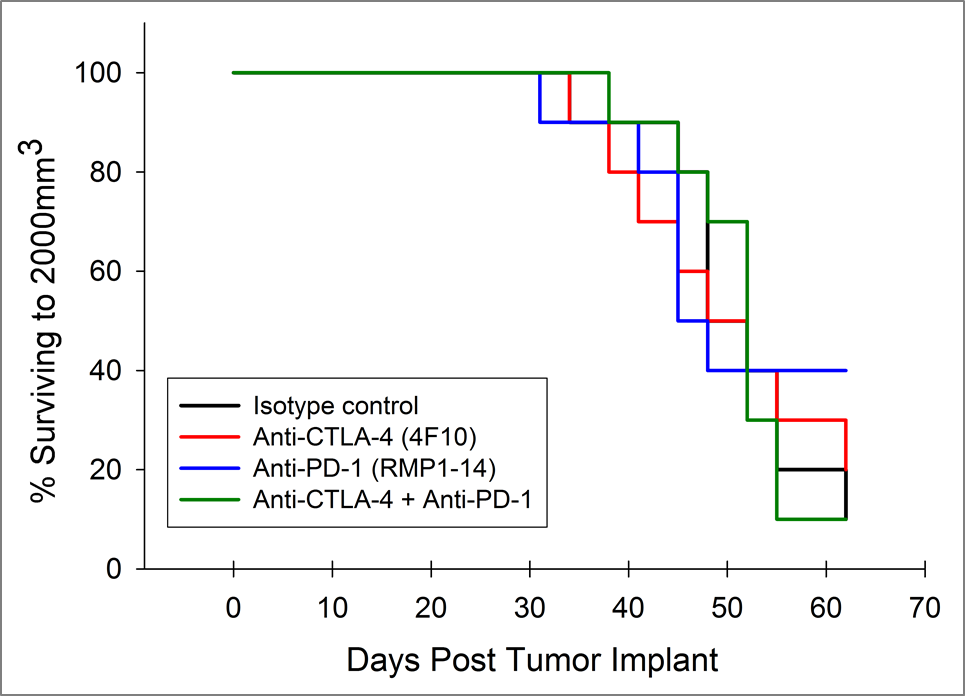
目前,针对CTLA-4、PD-1和PD-L1的T细胞检查点抑制剂抗体已被批准用于非胰腺癌。我们在Pan02模型中单独和联合检测了抗CTLA-4(克隆4F10)和抗PD-1(克隆RMP1-14)。植入后三天开始给药,平均组肿瘤负荷表明任何组均无明显活性(图2A)。通常情况下,免疫疗法可能只显示受治疗动物的一个子集的活性,因此个体动物图对检测这些应答者很有价值。然而,单独的动物图没有显示出在对照组中也没有观察到的试验药物的任何显著活性(图2B)。此外,根据生长反应数据,与对照组相比,治疗组的存活率没有改善(图3)。
免疫分析
我们想确定检查点抑制剂是否对Pan02肿瘤的免疫细胞浸润有影响。图4显示了总CD45+白细胞、CD4+T细胞、CD8+T细胞、调节性T细胞(Tregs)、单核细胞骨髓源性抑制细胞(M-MDSC)和粒细胞MDSC(G-MDSC)的免疫特征。
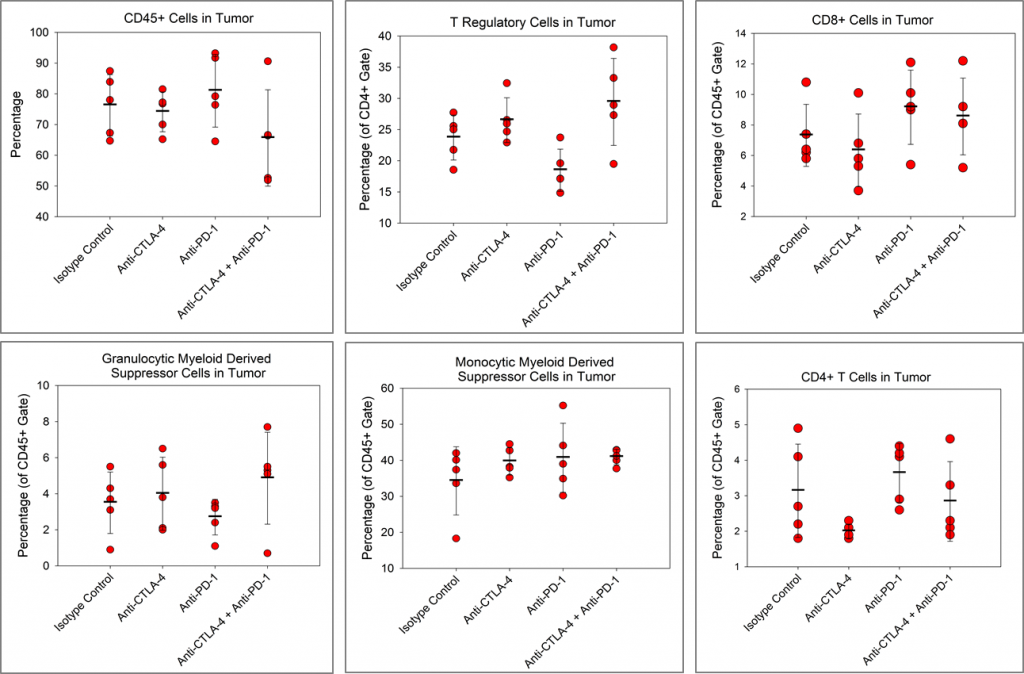
Although, there is a trend toward reduced CD4+ T cells in the anti-CTLA-4-treated group and a trend toward reduced Tregs in the anti-PD-1 treated group relative to isotype control, no other immune changes are observed. Despite checkpoint inhibitors alone having no apparent activity in the Pan02 model, there are published reports of checkpoint inhibitor activity in Pan02 tumors with radiation3 or a costimulatory agonist anti-CD40 antibody.4 Importantly, anti-PD-L1 strongly synergized with both anti-CD40 and radiation in those studies indicating that while Pan02 tumors are refractory to single agent checkpoint inhibitors, checkpoint inhibition can promote activity of combination partners.
联系我们to speak with one of our scientists to see how Pan02 or one of our other syngeneic models can be used for your next immuno-oncology study.
1Corbett TH等人,《C57BL/6小鼠胰腺两种可移植导管腺癌的诱导和化疗反应》。《1984年癌症研究》,第44卷:717-726。
2Wang Y等,小鼠胰腺癌细胞关键基因的基因组测序。2012当前分子医学。第12卷(3):331–341。
3Azad A等人,《PD-L1阻断增强胰腺导管腺癌对放疗的反应》。2017EMBO分子医学。第9卷:167–180。
4Luheshi NM等人,《CD40激动剂抗体对肿瘤微环境的转化与小鼠原位胰腺肿瘤模型中PD-L1阻断反应的改善相关》。2016肿瘤靶点。第7卷(14):18508-18520。
注:研究是根据适用的动物福利条例在AAALAC认可的机构中进行的