Determining preclinical CAR T-cell persistence by flow cytometry
在临床前研究和yaboapp体育官网开发期间,通常使用鼠标作为测试对象来研究轿厢(嵌合抗原受体)T细胞疗法。在这种环境中,免疫功能性的小鼠携带人来源的肿瘤负担,并用同种异体的人汽车T细胞给药。通过工程化的嵌合抗原受体引导了养护的嵌合抗原受体,以结合肿瘤细胞上的表面抗原(例如CD19)。由于工程化T细胞与肿瘤细胞结合,T细胞被激活并在MHC不受限制的庄园中杀死肿瘤细胞。临床前研究研究中yaboapp体育官网的小鼠遵循许多预定义参数,评估肿瘤生长和负担以确定汽车T细胞的功效(Learn more about how efficacy can be determined by bioluminescence imaging (BLI)。
A key success factor of the CAR T cell antitumor response is how long the CAR T cells exist after infusion into the host (persistence). Despite full functionality, it has been shown that poor persistence of CAR T cells can limit an effective antitumor response.1In the clinical setting, long-term remission in patients with hematological malignancies is associated with sustained persistence of CAR T cells.2共刺激信号思想影响T细胞扩张和持久性。yaboapp体育官网研究设计为包括4-1BB共刺激结构域的汽车构建体的效果的临床前研究已经与长车T细胞持久性,较慢且持续的效应功能以及更高比例的存储器T细胞有关。3.Assessing CAR T persistence in preclinical studies have implications in determining clinical success.
This technology spotlight will provide the reader with information about measuring CAR T cell persistence, using flow cytometry during the preclinical phase.
Mouse Models for CAR T Cell Candidate Evaluation
2017年9月,食品和药物管理局批准了第一台汽车T细胞疗法。细胞疗法由诺华州销售,被称为Kymriah。Kymriah也被称为tisagenlecleuceland was approved for children and young adults who no longer respond to standard therapies for B cell acute lymphoblastic leukemia. Kymriah is based on the work of Carl June, PhD., at the University of Pennsylvania in NSGTMmice.
nod.cg-PrkdcscidIl2rgtm1wjl./SzJ mouse, commonly known by the branded name, NODscid伽玛(NSG™),不要表达Prkdcgene nor the X-linkedIl2rggene. Thescid(severe combined immune deficiency) mutation is in the DNA repair complex protein Prkdc and renders the mice B and T cell deficient. These mice do not have complement nor natural killer (NK) cells and are deficient in cytokine signaling pathways. These deficiencies make the mice ideal and highly amenable to engraftment of human immune cells, including CAR T cells. The mice engraft a wide range of human tumors and are the gold standard for preclinical CAR T cell research.
在我们的yaboapp体育官网临床前肿瘤学部门, we can use any commercially available mouse strain for CAR T cell therapy and other adoptive cell transfer studies, including NSGTMmice and, under certain conditions, we can accept specialty mice as well.
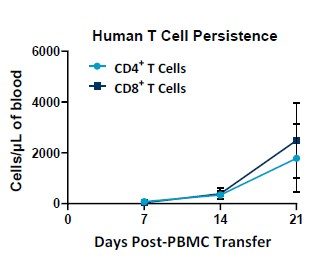
The PersistenceTTM来自临床前肿瘤的面板用yaboapp体育官网于产生纵向汽车T细胞持久性数据可以与全血的5uL一样少。它被格式化为具有绝对计数的Lyse / No洗涤测定。该面板具有灵活性,提供了在FITC,PE和APC通道中添加特定的防车探针的机会。来自杰克逊实验室(巴港,缅因州,美国缅因州)的NSG小鼠进行了2倍7.人类PBMC,全血3-,14-和21天后给药后,并经受持久性TMPanel evaluation.
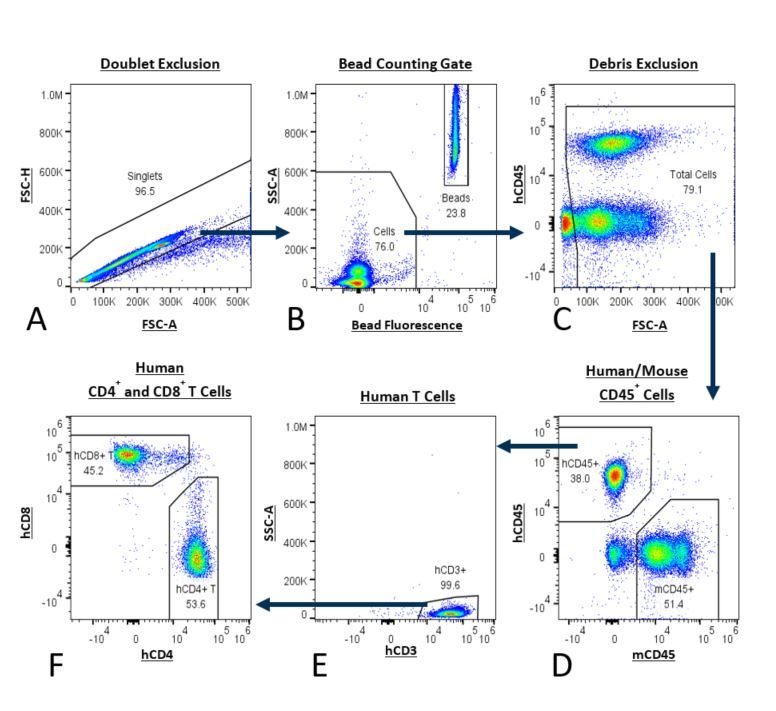
Table 1 describes the reagents used in the PersistenceTTM控制板。门控策略在图1中详述。
Antibody/Dye |
描述 |
|---|---|
mCD45 |
Mouse pan-hematopoietic cells |
hCD45 |
Human pan-hematopoietic cells |
hCD3 |
Human pan-T cell marker |
hCD4 |
Human CD4+T细胞标记 |
hCD8 |
Human CD8+T细胞标记 |
Counting Beads |
提供绝对数量 |
Viability Dye |
Dead cell exclusion |
Optional: CAR Specific |
FITC, PE or APC |
表1:persistencetTMPanel of antibodies and description of their use.
Longitudinal Immunophenotypic Characterization of CAR T Cells Using the PersistenceTTMPanel
In the临床肿瘤环境, CAR T cell expansion and persistence correlates with response and attaining remission in patients.4.Because of this observation, establishing reliable methods of tracking CAR T cell numbers is particularly important, not only in the estimation of effectiveness of CAR T cell therapy, but in terms of safety evaluation in the preclinical setting.Serious side effects of clinical CAR T cell therapy are noted, one of the most severe being cytokine release syndrome (CRS) and is associated with CAR T cell expansionin vivo。5.
The PersistenceTTM面板直接适用于随时间从小鼠取出的样品中的人轿厢T细胞数进行评估。由于整体血液要求较低,样品可以从同一鼠标中取出几次或使用小鼠的群组来实现研究目标。NSG小鼠可以放在长期持续时间研究中,用于重点轿车持久性评估。Persistencet如何的一个例子TM面板可用于测量NSG小鼠中的人T细胞随着时间的推移,如图2所示。
Improving CAR T Cell Persistence
车体内T细胞的持久性是一个代理er of long-term clinical efficacy of CAR T cell therapy. Studies have shown that certain CAR T cells, containing a CD28 costimulatory domain exhibit increased expression of T cell exhaustion-related genes, while the 4-1BB (TNFSF9) costimulatory domain with the same antigen specificity reduced the exhausted phenotype. This may help explain why in clinical trials of patients with relapsed or refractory Acute Lymphoblastic Leukemia, CAR T cells expressing a CD28 domain have been reported to persist for up to 3 months, while CAR T cells with a 4-1BB domain persist for up to 5 years, and more than 6 months in almost all of the cases that could be evaluated.6.
通过世代汽车T细胞
Car T细胞继续通过修饰嵌合抗原受体而发展。每个汽车T细胞都有一个SCFV(单链片段变量)。SCFV是重链(VH)和轻链(VL)结构域的可变区域的组合。SCFV是VH和VL的融合蛋白,免疫球蛋白,与10至25个氨基酸之间的短接头肽连接。
First generation CARs have an extracellular binding domain, a hinge region, a transmembrane domain and one or more intracellular signaling domains. All CAR T cells have the CD3 ζ chain domain for intracellular signaling and is the primary transmitter of T cell activation signals. The addition of a co-stimulatory domain (CD28 or 4-1BB) defined second generation CARs. The aim was to improve T cell proliferation, cytokine secretion and in vivo persistence.
Preclinical data shows that third generation CARs have improved effector function and longer in vivo persistence in contrast to second generation CARs. Third generation CARs have multiple co-stimulatory domains (CD28-41BB or CD28-OX40). Armored CARs and TRUCKs (CARTcellsr编辑为universalcytokinekilling)是有时用于第四代汽车T细胞的名称。第四代汽车T细胞可以包括增强T细胞扩张,抗肿瘤活性和持久性的因素。7.
Regardless of the CAR or TRUCK generation, the PersistenceTTMPanel provides an excellent tool for short- or long-term monitoring of CAR T cell persistence in vivo in the preclinical setting.
Summary
使用Persistencettm面板,测量汽车T持久性简单。我们的流式细胞术面板允许从少量血液中确定纵向汽车T持久性。我们的人类Compttm小组(表2)是如何将其他合格的面板添加到临床前的汽车T研究中,以产生全套流式细胞术数据。yaboapp体育官网
Antibody/Dye |
描述 |
|---|---|
hCD45 |
Human pan-hematopoietic cells |
hCD3 |
Human pan-T cell marker |
hCD4 |
Human CD4+T细胞标记 |
hCD8 |
Human CD8+T细胞标记 |
HCD25 |
Human regulatory T cell marker |
hFoxP3 |
Human regulatory T cell marker |
hPD-1 |
Human T cell inhibitory signaling protein |
hCD69 |
激活标记 |
ki-67. |
Proliferation marker |
Viability Dye |
Dead cell exclusion |
Table 2.人类的个人标记TMLeukocyte Panel.
了解有关Persistencet的更多信息TMPanel, or one of our other flow cytometry panels, can be incorporated into your preclinical oncology research and to learn more about our extensive flow cytometry programcontact the scientists.
Please note that allanimal care and usewas conducted according to animal welfare regulations in an AAALAC-accredited facility with IACUC protocol review and approval.
1Song DG, Ye Q, Carpenito C, Poussin M, Wang LP, Ji C, Figini M, June CH, Coukos G, Powell DJ Jr. In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T cells is enhanced by costimulatory signaling through CD137 (4-1BB).
Cancer Res. 2011 Jul 1;71(13):4617-27.
2Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17.
3.Long啊,Haso WM,Shern JF,Wanhainen Km,Murgai M,Ingaramo M等人。4-1BB共刺激改善了通过嵌合抗原受体的滋补信号传导引起的T细胞耗尽。Nat Med。2015; 21:581-90。
4.波特DL,黄WT、弗雷NV莱西科幻,肖PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139.
5.Santomasso B,Bachier C,Westin J,Rezvani K,Shpall EJ。汽车T细胞疗法的另一边:细胞因子释放综合征,神经系统毒性和金融负担。
Am Soc Clin Oncol Educ Book. 2019 Jan;39:433-444.
6.Maus MV and June CH. Making Better Chimeric Antigen Receptors for Adoptive T-cell Therapy. Clin Cancer Res. 2016 Apr 15; 22(8): 1875–1884.
7.Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015;15(8):1145-54.