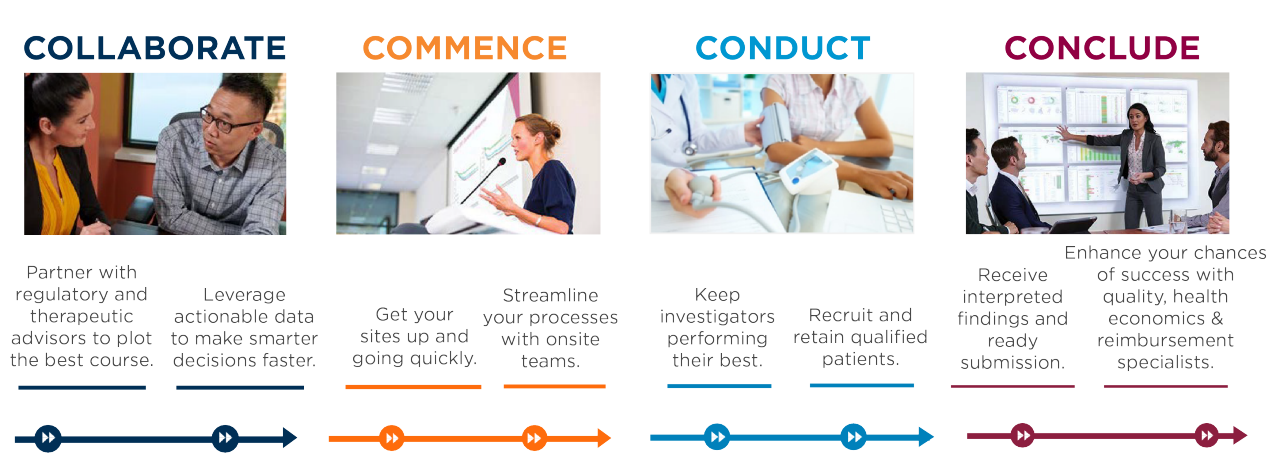
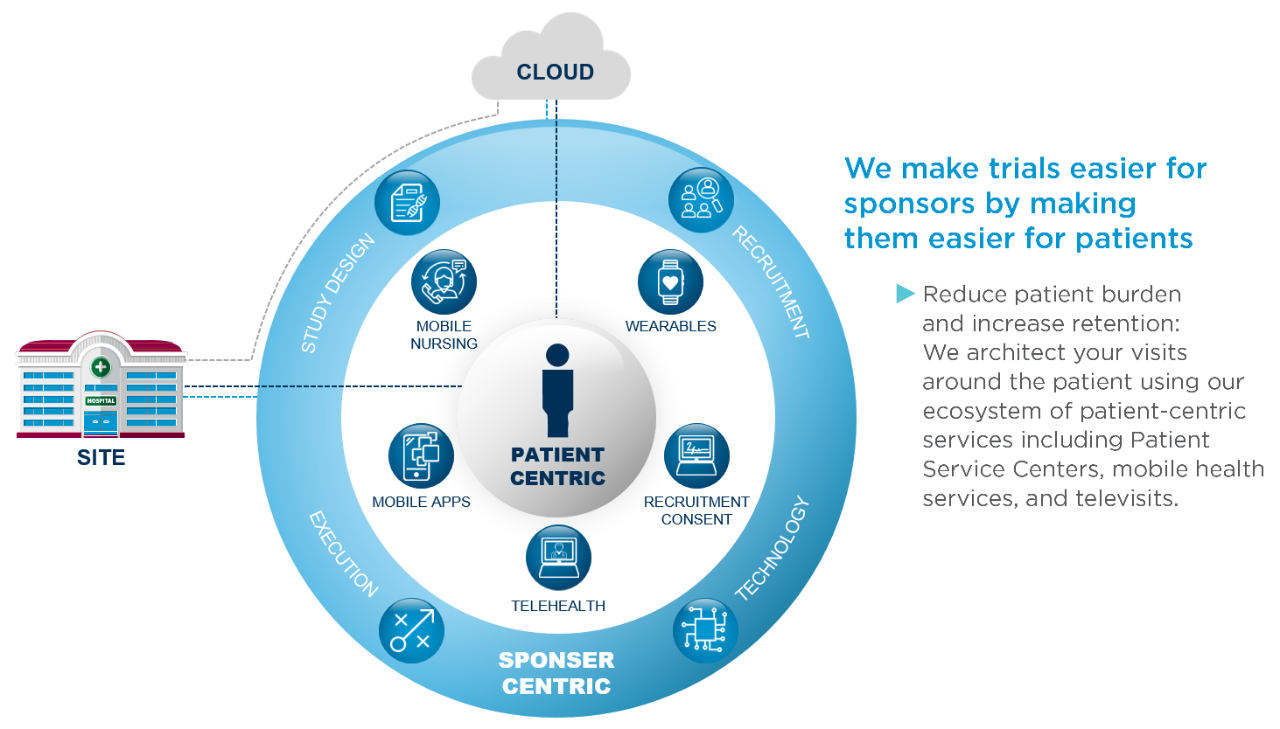
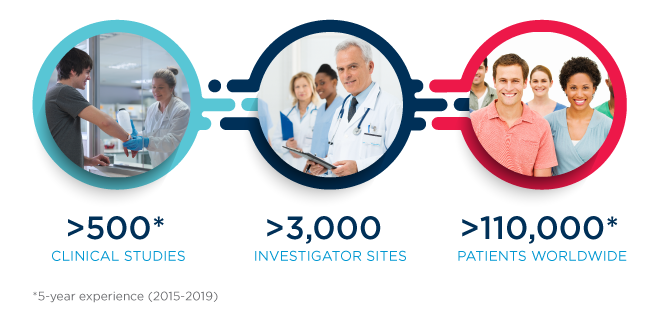
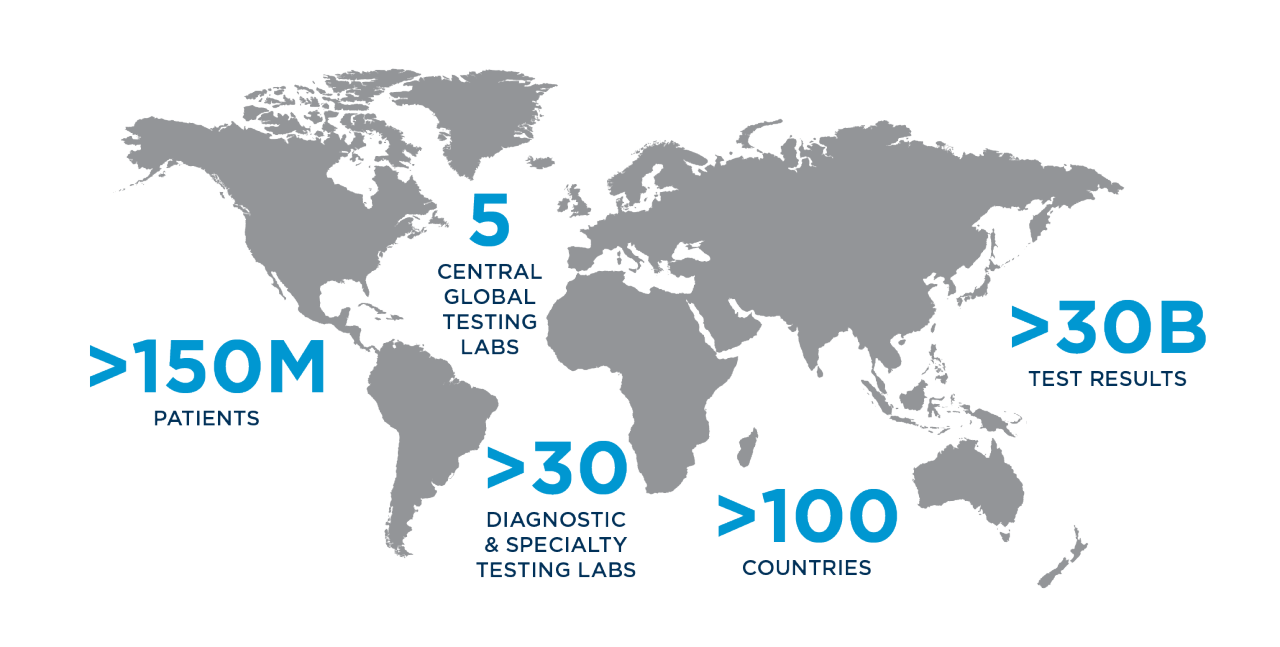
Once you receive approval to advance your device to the clinical stage, you will have many new questions to address, such as:
- 收集和报告最好支持我的审判目标是什么?
- How can I identify and qualify preferred sites to ensure enrollment and study compliance success?
- 什么是最好的患者人口,如何激励患者参加我的审判?