深入研究肿瘤浸润性T细胞免疫表型
AUTHOR:
David Draper, PhD | Associate Director, Scientific Development
DATE:
August 2019
流式细胞术使用大抗体面板的优点是检测更多的细胞亚群。当需要一个全面的数据集,但可用于分析的肿瘤材料有限时,这种能力尤其重要。此外,含有大量抗体的面板也可用于对少数亚群进行深入的免疫表型分析,或对单个亚群进行更深入的分析。在这个技术焦点中,我们通过展示使用新的扩展CompT生成的数据来展示后一种能力™ 面板,一个16色面板,对T细胞活化和分化进行流式分析,其水平高于Covance服务组合中的任何其他面板。
The Expanded CompT™ panel builds upon the CompT™ panel, our most popular standard panel to examine CD4+和CD8+T细胞。这个改进的小组增加了效应和记忆T细胞标记,加上四个额外的标记分析T细胞活化和衰竭。表1描述了扩展CompT的组件™ 小组,并使用未经治疗的小鼠MC38结肠癌,图1说明了其门控和分析策略。
表1:扩展的Compt™面板抗体和其实用程序的描述
| Antibody/Dye | 说明 |
|---|---|
| CD45细胞 | 泛免疫细胞标志物 |
| CD3. | Pan-T cell marker |
| CD4. | CD4.+ T cell marker |
| CD8. | CD8 + T细胞标记 |
| 福克斯P3 | 法规T cell marker |
| CD25型 | 调节性T细胞标志物/IL-2受体 |
| CD44细胞 | Activation/Memory marker |
| CD62L型 | Naïve T cell/Memory marker |
| Ki-67 | Proliferation marker |
| CD69型 | T细胞活化标志物 |
| PD-1型 | T cell activation/Exhaustion marker |
| 滞后3 | T cell activation/Exhaustion marker |
| TIM-3 | T细胞疲惫标记 |
| ICOS | T细胞活化标志物 |
| 颗粒酶B | 抗肿瘤细胞毒性标志物 |
| Viability Dye | Dead cell exclusion |
| 扩展组件™ 可定制包括NK/NKT细胞标记物(CD49b/CD335)以使颗粒酶B和活化标记物在这些亚群中表达。 | |
Expanded CompT™ Panel Gating Strategy
正如所有Covance T细胞板,分析开始with dead cell exclusion and subsequent CD45+immune cell delineation to gate on CD3+T cells (not shown). Figure 1A displays the downstream endpoints of CD4+和CD8+T细胞分析在Compt™和扩展的Compt™面板之间共用。这些包括CD69和PD-1,其在T细胞活化时上调。它们的表达与已耗尽的T细胞表型相关。[1]另一个共享端点是CD8+通过使用Ki-67表达作为替代标记物来提供T细胞增殖。最后,CD4+检测T细胞以量化辅助性T细胞和调节性T细胞(Tregs)。图1B和1C说明了用于扩展CompT的添加端点™ 面板,下面将进一步介绍。
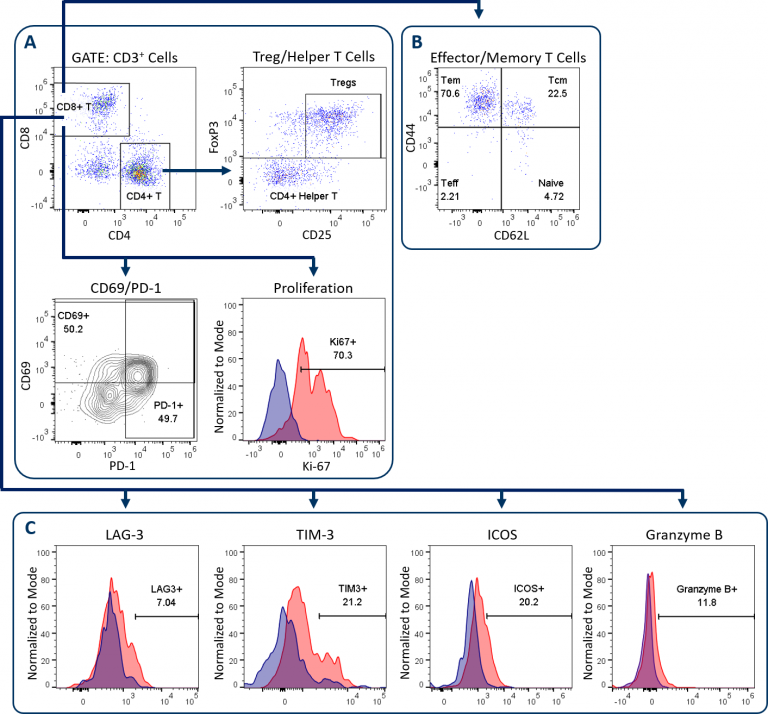
Effector/Memory T Cell Analysis
Analysis of effector and memory CD8+T cell differentiation is shown in Figure 1B. Conversion of T cells to a memory phenotype is important for the development of lasting immunological response to rechallenge in the context of both infection and cancer pathogenesis. CD44 and CD62L analysis enables the delineation of T cells into four differentiation states. These include naïve or inactivated T cells, activated effector T cells (Teff), effector memory (Tem) and central memory (Tcm) subsets. Tem and Tcm subsets can circulate but have tendencies to reside in non-lymphoid and lymphoid tissues, respectively.[2]Recent reports have demonstrated that both of these subsets have distinct roles in the anti-tumor response. A third resident memory (Trm) population has more recently been described as playing an important role in controlling tumor growth in a variety of models and can be delineated using CD103, among other markers.[3]扩展组件™ 面板可以定制为包括Trm细胞的分析。
T细胞活化、衰竭和颗粒酶B分析
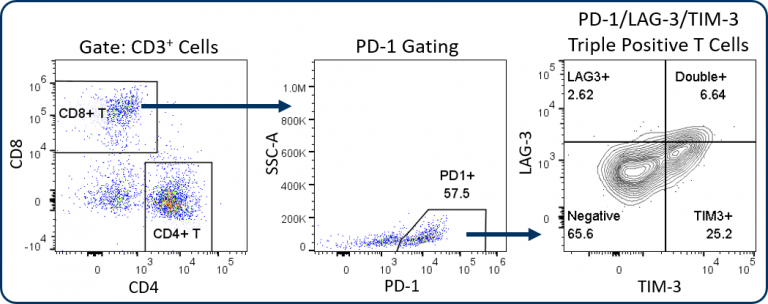
The Expanded CompT™ panel includes ICOS, LAG-3, TIM-3, and granzyme B analysis, which are four intensively investigated biomarkers for T cell functionality (Figure 1C). The analysis of these targets alone and in combination can provide insight into the anti-tumor potential of CD8+ T cells. Evidence supports a co-stimulatory and anti-tumor role for ICOS receptor signaling, thus making ICOS an attractive therapeutic target.[4] Granzyme B is often used as a biomarker for cytolytic activity and can correlate with CD8+ T cell anti-tumor responses. Conversely, PD-1, LAG-3 and TIM-3 are inhibitory receptors and while the expression of these three receptors has been linked to T cell exhaustion, a growing body of data suggests heterogeneity among sub-populations exists within the exhausted PD-1 expressing CD8+ T cells.[5] This heterogeneity correlates with the expression pattern of these inhibitory receptors. This profile can help define different sub-populations that have distinct potential to be re-invigorated to proliferate and/or lyse tumor cells.[6] Figure 2 illustrates how the Expanded CompT™ panel can quantify cells with double and triple positive expression for inhibitory receptors and provide insight into the heterogeneous PD-1 expressing T cell subset and its functionality. Numerous other T cell activation and inhibitory receptors have been described and implicated in influencing tumor immune responses; these include TIGIT, OX-40, CD137, CTLA-4, and others. Covance has experience analyzing many of these markers in离体tumor analysis. With minimal developmental efforts, the Expanded CompT™ can be customized to meet your unique pre-clinical needs.
定制–NK/NKT细胞分析和更多选项
Covance can configure custom panels with up to 18 colors, which creates options for the MI-Expanded CompT™ panel. In addition to substituting or adding different T cell activation/exhaustion markers as described in the previous section, NK/NKT cell analysis is a potentially valuable endpoint. This is enabled by the addition of CD49b/CD335 markers to the panel (Figure 3).
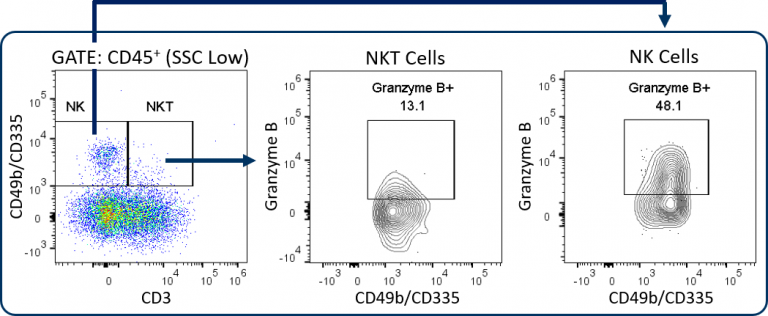
NK and NKT cells are an important source of IFNγ, have indirect effects on enhancing CD8+ T cell anti-tumor responses, and can directly lyse tumor cells by releasing cytolytic granules such as granzyme B.[7,8] Other options include IFNγ, TNFα, or other cytokine analyses for a more in depth profile of PD1+ and PD1– CD8+ T cells. Or add CD103 analysis to examine resident memory T cells for a deeper memory T cell profile in the tumor. Covance’s team has extensive experience developing custom flow cytometry services. To learn more about how the Expanded CompT™ panel can be incorporated into it into your preclinical research, contact the scientists at Covance.
1江,Y,Y。李和B。“朱先生。”肿瘤微环境中的T细胞衰竭。”细胞死亡与疾病6.6(2015):e1792。
2克列班诺夫、克里斯托弗A、卢卡·加蒂诺尼和尼古拉斯P。Restifo。”肿瘤免疫学和免疫治疗中的CD8+T细胞记忆。”免疫学评论211.1(2006):214-224
3Mami-Chouaib,Fathia等。“常规记忆T细胞,肿瘤免疫学中的关键组分。”Journal for immunotherapy of cancer6.1(2018):87。
4阿马托尔、弗洛伦特、劳伦特·戈维尔和丹尼尔·奥利弗。”诱导型共刺激因子(ICOS)是一种潜在的抗癌治疗靶点治疗靶点专家意见22.4 (2018): 343-351.
5Miller, Brian C., et al. “Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade.”自然免疫学20.3 (2019): 326.
6熊慧忠等:“抑制性受体的共表达丰富了小鼠同基因肿瘤模型中活化和功能性CD8+T细胞。”Cancer immunology research7.6(2019):963-976。
7Zhu, Yanting, Bo Huang, and Jue Shi. “Fas ligand and lytic granule differentially control cytotoxic dynamics of natural killer cell against cancer target.”Oncotarget7.30 (2016): 47163.
8Zhao, Jie, et al. “Polyclonal type II natural killer T cells require PLZF and SAP for their development and contribute to CpG-mediated antitumor response.”美国国家科学院学报111.7(2014):2674-2679。
Note: Studies were performed in accordance with applicable animal welfare regulations in an AAALAC-accredited facility