Biomarker Solutions, Designed Around You
Multi-disciplinary and integrated solutions to meet your biomarker strategy goals
Personalized biomarker support to help drive your development from bench to commercialization
协作,科学设计和解决问题,适合您的独特要求
Deep expertise and insights to drive your preclinical and clinical – including exploratory - biomarker programs – even into commercialization
创新,最先进的平台和全球实验室测试能力

生物标志物在当今药物开发的震中和追求个性化药物,这占2018年的超过40%的FDA批准1。它们有助于确定疾病生物学,驱动患者鉴定和分层,识别作用机制,并在其他方面理解药物的药效学方面。
A Clear Path Forward for Efficient Drug Development
为了充分实现生物标志物战略的好处,您需要早期见解,集中专业知识和清晰的蓝图,包括最先进的技术和平台。它始于深入了解生物标志物在您的程序中的作用,拟议的应用程序,以及哪些工具将帮助您实现目标。
Selecting the most appropriate regulatory environment for your biomarker program must also be considered. Rapid development, validation and – when needed – transfer of your assay will optimize your timeline and financial impact.
1Personalized Medicine Coalition. Personalized Medicine at FDA: A Progress & Outlook Report, 2018
An Approach as Unique as Your Molecule
Led by ourBiomarker Solution Centerteam of PhDs focused on key therapeutic areas, the design for your biomarker program is informed by industry insights and deep specialized knowledge around three key areas:
生物标志物的作用
- 预测
- Pharmacodynamics
- 监测
- Prognostic
- 安全
- 易感性/风险
- 诊断
Intended Use
- Mechanism of Action Identification
- Surrogate Endpoint
- Patient Identification / Stratification
- Inclusion/ exclusion
- 病人护理
- 同伴的诊断
工具
- 下一代测序
- 流式细胞仪
- 免疫组织化学
- Immunoassays
- 基于细胞的测定
- LC-MS
- 解剖病理学
The result is a customized approach that takes into account the entire program, platforms and timely progression toward your milestones.
在Covance和Labcorp,广泛的全球实验室测试能力,从标准临床实验室和安全测试到深奥和复杂的生物标志物测试,构成了4,500多个测定和为您的生物标志物开发新测定的能力。无论您的计划是否需要探索性/适用性的测定或GLP / GCLP和CAP / CLIA调节环境,您将在您的计划中获得高效的安置,并随着您的监管需求转变为经济转移。
Biomarker Solutions from Bench to Commercialization
Seeing the entire drug development process is key to understanding how biomarker data utilization shifts as your program matures – and responding rapidly to keep your timelines intact.
无论您是积极规划CDX登记审判还是仍然试图确认您的生物标志物候选者预测对治疗的反应,诊断连续体的整体概述都可以避免在监管监督,数据完整性和开发时间表中的差距。
早期参与届及诊断开发服务团队可以通过商业化从概念中获得工业领先的专业知识。
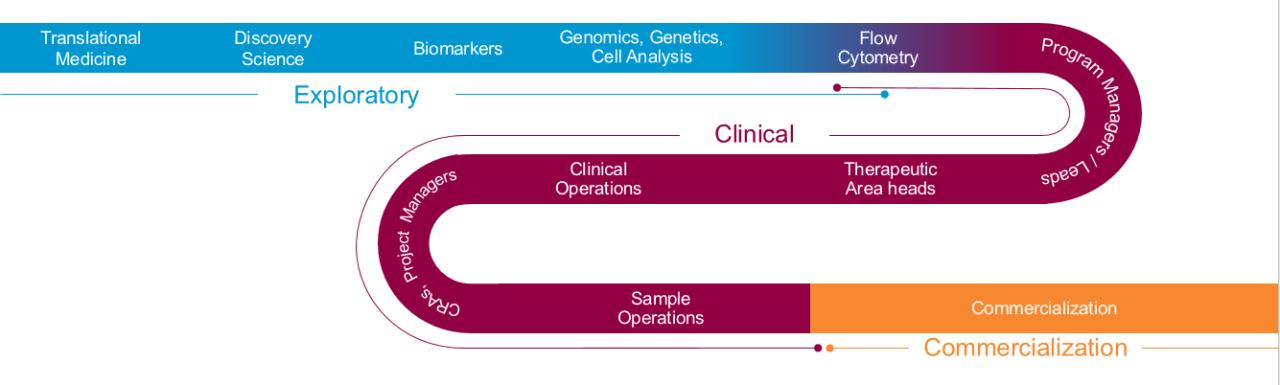
- Exploratory- 从命的目标开始 - 是否探索或支持临床试验 - 以及我们的团队翻译生物标志物专家to establish the right pathway to get you the answers you need, when and how you need them.
- 临床– Progress into the clinic and speed patient recruitment by relying on a team that can help you navigate to the right laboratory within the Covance and the extended LabCorp specialty testing network.
- Commercial- 如果您的预测生物标志物进入a,则防止延迟伴侣或互补诊断。确信您的诊断和治疗将通过与支持超过60%的FDA批准的伴侣和市场互补诊断的团队合作,在“第一天”上商业准备。
