全球监管事务和产品开发咨询
Access critical support for your end-to-end strategy’s registration and commercialization.
导航今天复杂环境的指南
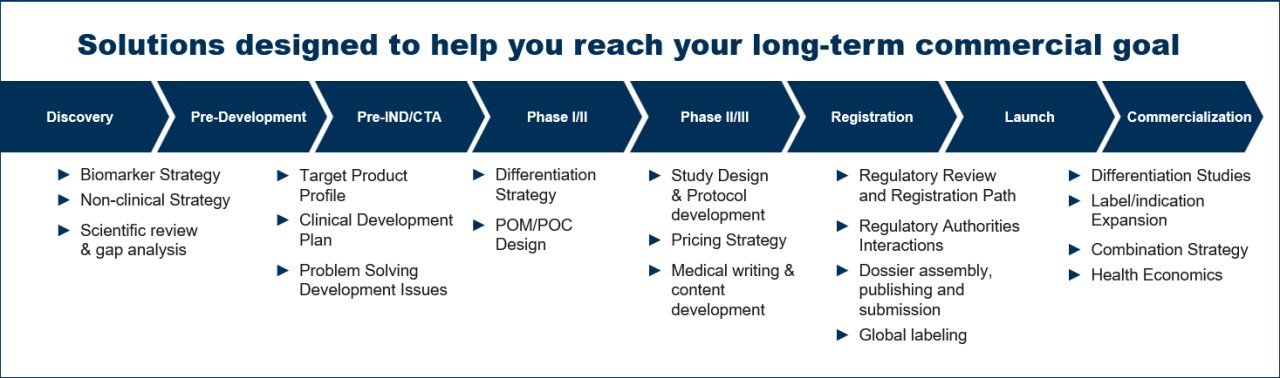
达到商业化是一个复杂和迭代的过程。在不断发展的药物发展景观中取得成功,您必须:
- Find the fastest path for reviewing your molecule
- Traverse an intricate development process
- Reduce inefficiencies at multiple stages
- 确保商业吸收
您需要一个经验丰富的合作伙伴,他们知道如何帮助您达到长期商业目标。依靠Covance提供创新的监管战略 - 在您发展的任何阶段。
一个倡导者,与您的资源保持一致
无论您的未来下一步还需要对您的非临界战略或注册和商业化还是建议,您是否需要对您的下一步的咨询,无论您的公司规模或产品开发阶段如何,我们都可以满足您的监管需求的各个方面。
加强您的全球战略,代理商会议和互动
Integrated global drug development has become the norm where biotech and pharmaceutical companies can now achieve near simultaneous regulatory approval of products worldwide. You need an experienced partner who can review your data, develop your submission and act as your guide through critical agency meetings. Whether you are following the traditional submission route or seeking fast-track or orphan drug designation, we have on-the-ground specialists who can work directly with your team and, if needed, even act on your behalf at agency meetings.

No matter where you are seeking approval, we can help you work with the FDA, EMA, MHRA, NMPA and PMDA and design a regulatory solution.
Access more than regulatory support
Optimizing the clinical and commercial value of your product starts with a strong and early strategy. OurMarket Access and Phase IV Solutionscan assess your market landscape, conduct research and communicate findings to help demonstrate value, augment your team to help communicate your message, and support your patients, customers and overall access strategy.
包含全球开发能力
尽量减少发展中的空白空间,并通过我们的专利生命最大限度地提高您的专利生命分子开发组. You’ll get strategic continuity from early clinical development to Phase IV with a core team of experienced physicians and project managers who will forge your path through each stage of the drug development process. As you bring in our subject matter experts when needed, you’ll increase continuity between strategy development and study execution, driving your portfolio with less overhead.
Derisk your development
创建一个全面的战略,确定新的efficiencies with a holistic view of your program. We can help you plan the best path ahead – from the very beginning – and offer full coverage at every stage. From strategy, audit, submission, and publishing to post-licensing support, let us support your molecule with multiple perspectives. Whether you need an extension of your team to provide advice or someone to represent your product at meetings, we can provide extended solutions across our enterprise to help you efficiently navigate regulatory approvals.
