生物标志物解决方案,设计为您
多学科和综合解决方案,以满足您的生物标志物战略目标
个性化的生物标志物支持,帮助您从长凳到商业化的发展
Collaboration, scientific design and problem-solving that fits your unique requirements
推动您的临床前和临床 - 包括探索性生物标志物计划的深度专业知识和见解 - 甚至进入yaboapp体育官网商业化
Innovative, state-of-the-art platforms and global laboratory testing capabilities

生物标志物are at the epicenter of today’s drug development and the pursuit of personalized medicines, which accounted for more than 40% of FDA approvals in 20181. They help define disease biology, drive patient identification and stratification, identify mechanism of action and understand the pharmacodynamic aspects of a drug, among other aspects.
高效药物开发的明确道路
充分认识到生物标志物挺起的好处egy, you need early insights, focused expertise and a clear blueprint that includes the most advanced techniques and platforms. It begins with a deep understanding of the role of biomarkers in your program, the proposed application, and which tools will help you achieve your goals.
还必须考虑为生物标志计划提供最合适的监管环境。快速发展,验证和 - 当需要时 - 转移您的测定将优化您的时间表和财务影响。
1个性化医学联盟。FDA的个性化医学:2018年进度与展望报告
一种作为分子独特的方法
由我们领导生物标志物解决方案中心PHDS团队专注于关键治疗领域,您的生物标记计划的设计是通过行业见解和深度专业知识的三个关键领域(:
Role of Biomarker
- Predictive
- 药效学
- Monitoring
- 预后
- Safety
- Susceptibility/ Risk
- Diagnostic
有可能的使用
- 行动鉴定机制
- 代理终点
- 患者识别/分层
- 纳入/排除
- Patient Care
- 伴侣诊断
Tools
- Next Generation Sequencing
- Flow Cytometry
- Immunohistochemistry
- 免疫测定
- Cell-based Assays
- LC-MS
- Anatomic Pathology
结果是一种定制的方法,它考虑了整个程序,平台,及时进步对您的里程碑。
Across Covance and LabCorp, extensive global laboratory testing capabilities, ranging from standard clinical laboratory and safety testing to esoteric and complex biomarker testing, constitute more than 4,500 assays and the ability to develop new assays for your biomarker. Whether your program requires exploratory / fit-for-purpose assays or GLP/GCLP and CAP/CLIA regulated environments, you’ll get efficient placement of your program and cost-effective transfer as your regulatory requirements shift.
生物标志物解决方案from Bench to Commercialization
看到整个药物开发过程是了解生物标志物数据利用率如何随着程序的成熟方式转变的关键 - 并迅速响应以保持您的时间表完好无损。
Whether you’re actively planning a CDx registration trial or still seeking to confirm that your biomarker candidate is predictive of response to therapy, a holistic overview of the diagnostic continuum can avoid gaps in regulatory oversight, data integrity and development timelines.
Early engagement with the Covance Diagnostic Development Services team provides access to industry-leading expertise in drug and diagnostic co-development from concept through commercialization.
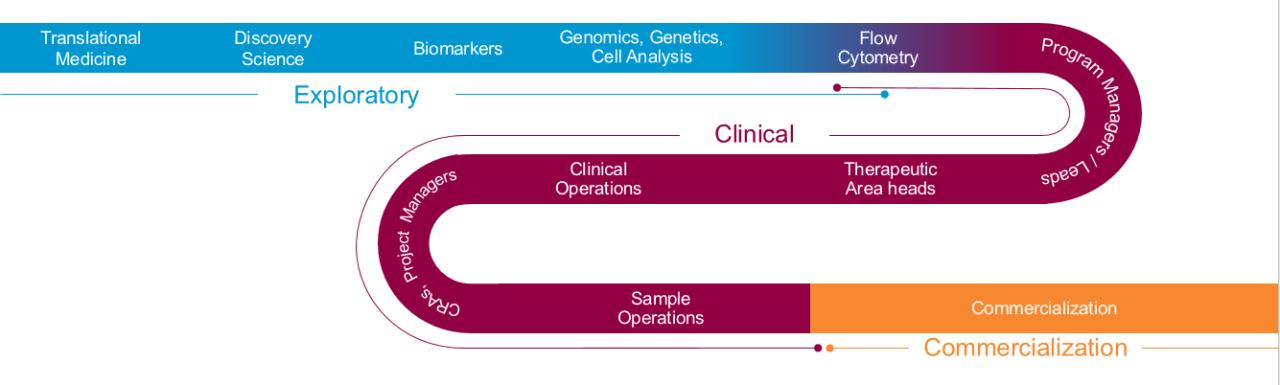
- 探索者– Start with your target goal in mind – whether exploratory or in support of clinical trials – and team with ourtranslational biomarker experts建立正确的途径,为您提供所需的答案,何时以及如何需要它们。
- Clinical- 通过依靠一个可以帮助您导航到Covance内的正确实验室和扩展Labcorp专业测试网络的团队进入诊所和速度患者招聘。
- Commercial– Prevent delays if your predictive biomarker advances to acompanion or complementary diagnostic. Be confident that your diagnostic and therapy will be commercially ready on “day one” by partnering with a team that has supported more than 60% of the FDA-approved companion and complementary diagnostics on the market.
