Models for pancreatic cancer
AUTHOR:
Erin Trachet, Sr. Scientific Advisor, Oncology / Sr. Manager, Proposal Development
日期:
2017年9月
超过90%的胰腺癌被归类为导管腺癌,在西方世界,胰腺癌是癌症相关死亡的第四个主要原因。胰腺癌的预后极差,5年相对生存率为5%,中位生存率为3.5个月的患者,患者不可切除肿瘤的患者.1不幸的是,胰腺癌的发病率一直在上升5年生存率没有改变。手术切除是唯一可能的疗效治疗,但只有10%的患者均已诊断为这一点,这是一种选择,也是有资格进行手术的最终复发的选择。与许多其他类型的癌症一样,胰腺癌在没有任何症状的情况下默默地生长。在大多数情况下,在癌症在胰腺外部生长到其他近端组织和/或转移之前,不会进行诊断。这些患者留下了很少有意义的选择。因此,胰腺癌治疗胰腺癌的有效新疗法。
For the last 15 years, patients diagnosed with advanced stage pancreatic cancer are given gemcitabine (Gemzar®) as the standard first line treatment. Preclinically, we use gemcitabine as our standard of care to provide a benchmark to our clients looking to surpass current clinical treatment options or to combine with novel therapies; such as targeted agents and immune-modulators.
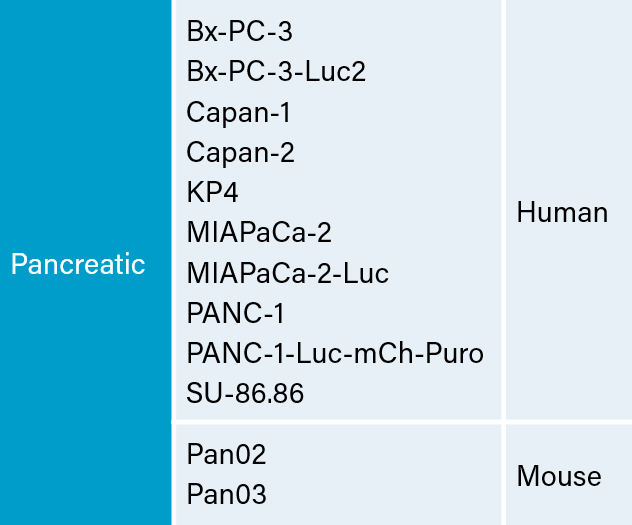
There are several human and murine pancreatic cell lines available to the preclinical cancer research community to aid in the development of novel therapies. Covance has a large panel of pancreatic lines ready for testing (see Table 1). We have optimized and characterized the subcutaneous (SC) growth for several of these models and evaluated their response to gemcitabine treatment.
PANC-1
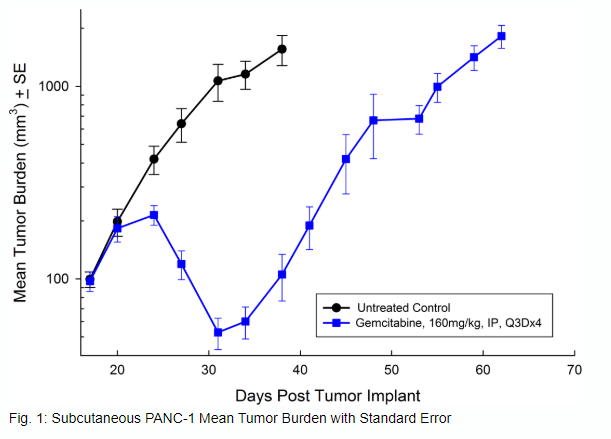
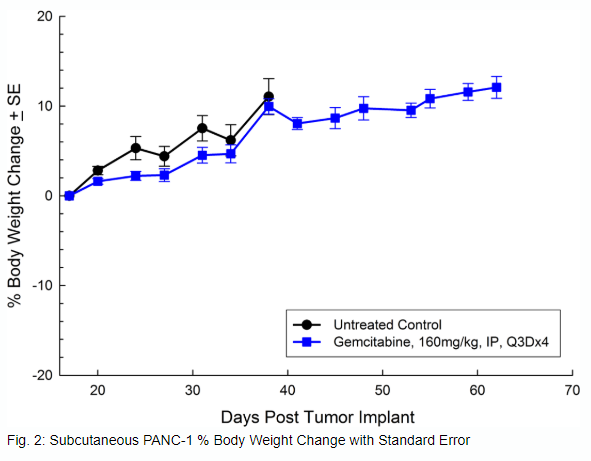
Panc-1被孤立于一个56岁的白种人男子,胰腺导管腺癌。皮下肿瘤生长可靠且一致,肿瘤体积每5天加倍,并且通常在植入后约29天内达到评价大小(〜750mM3)。用吉西他滨(160mg / kg)治疗耐受良好的耐受性,并产生统计学上显着的肿瘤生长延迟,但不是疗效(见图1和2)。
蜡蜡座2.
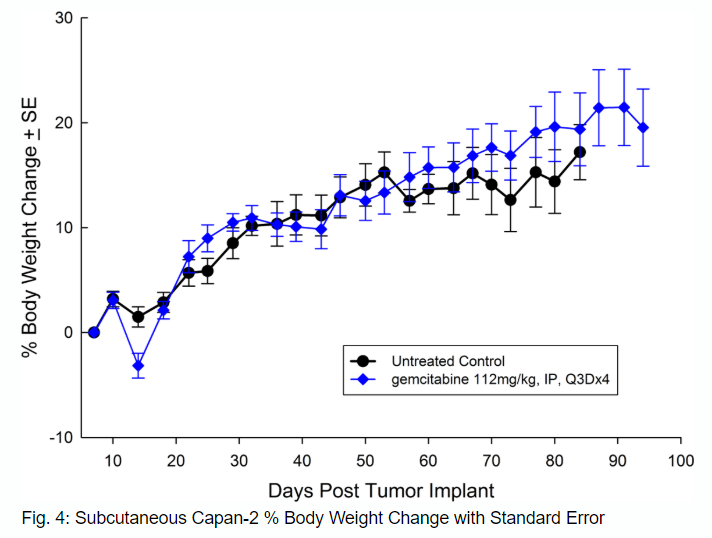
从56岁的白种人Capan-2、孤立with pancreatic ductal adenocarcinoma has subcutaneous tumor growth that is a bit slower than PANC-1 with a tumor volume doubling time of about 12 days and the time to reach evaluation size (750mm3) of about 36 days post implant. Treatment with gemcitabine (112mg/kg) is well tolerated and produces statistically significant tumor growth delay, with a large number of tumor free survivors (see Figures 3 and 4).

BX-PC-3
BX-PC-3从一个61岁的白种人女性中分离出胰腺癌。皮下肿瘤生长导致植入后约38天的肿瘤体积倍增时间约16天,达到评价大小(750mm3)。用吉西他滨(80mg / kg)治疗耐受良好但不能产生统计显着的肿瘤生长延迟(见图5和6)。

MIAPaCa-2
MIAPaCa-2 was isolated from a 65-year-old Caucasian man with pancreatic carcinoma. This model demonstrates very aggressive subcutaneous tumor growth with a tumor volume doubling time of about 3 days and the time to reach evaluation size (750mm3) of about 21 days post implant. Treatment with gemcitabine (160mg/kg) is well tolerated and produces statistically significant tumor growth delay, with one tumor free survivor observed in the study (see Figures 7 and 8).


与对护理标准的临床反应一样,治疗响应呈呈现在高度依赖于所使用的肿瘤线。yaboapp体育官网有许多因素可以为吉西他滨治疗有助于响应或缺乏。然而,在相同的指示中横跨几种不同的肿瘤系列可以提供有关新治疗在诊所有效程度的关键信息。
Pleasecontact us如果您有兴趣讨论我们的任何胰机质。
1Siegel R. L., Miller K. D. & Jemal A. Cancer statistics, 2015. CA Cancer J Clin 65, 5–29 (2015). [PubMed]
Note: Studies were performed in accordance with applicable animal welfare regulations in an AAALAC-accredited facility