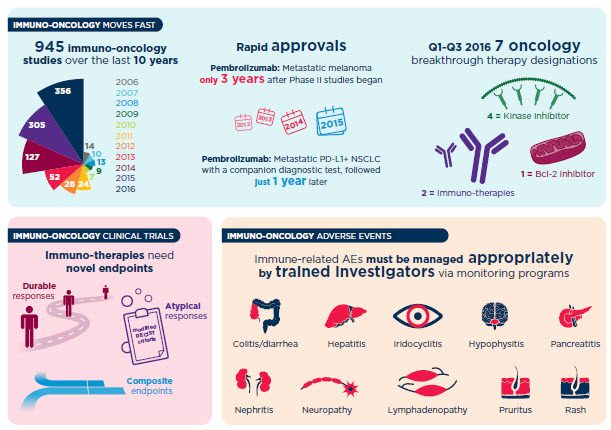
免疫肿瘤临床试验开发
加速你的immuno-oncology leverag研究ing scientific and operational expertise.
复杂的免疫肿瘤药物发展需要创新,承诺和专业知识
使用全面的临床前模型和生物标识识别策略,开发您的新型代理和伴侣诊断yaboapp体育官网
Optimize efficiency using advanced proprietary analytical platforms
Build lasting partnerships from proof of concept to market access with experienced investigators from Africa, the Americas, Asia-Pacific and the EU
Access the latest免疫脑肿瘤,白皮书,信息表,博客和案例研究.
Precision medicine. Advanced diagnostics.
我们的行业更深入地了解免疫系统在癌症中的参与情况下发现了癌症治疗的新方法。您需要快速筛选并识别单独和组合的安全和有效的分子,以便快速前进到下一个里程碑。利用我们的深度科学专业知识和精密药物经验,支持您的测试需求,并在创新的试验设计上执行,以实现您的独特疗法的成功。
无论是帮助您找到联合治疗,识别和分层生物标志物,发展伴侣诊断还是揭示市场机会的最佳化合物,我们的IO体验和xinyabo体育可以通过临床试验和批准后活动来提供从发现的解决方案,以在任何阶段推进您的开发。
Innovative informatics. Applied analytics.
Addressing the increased complexity of immuno-oncology drug development and the explosion of clinical and operational data requires new approaches to trial design and operational execution. Overcome inherent challenges by applying our multipronged approach that includes identification of premier sites, rapid startup and ongoing analysis of clinical data.
与我们这样的专有工具central laboratory database—with more than 40% of market data—along with LabCorp’s deep resource of patient aggregated and longitudinal data and theXcellerate®临床Trial Optimization Platform, you can leverage the latest analytical solutions to minimize risk, increase speed of execution and provide quality data to drive your decision making during your immuno-oncology clinical trials.
Enabling collaboration in an ever-changing environment.
Our industry’s ecosystem continues to shift. Rapidly advancing science, the nature of development and the commercialization process along with an increasing focus on patient centricity must be recognized to effectively facilitate progress.
Collaboration and engagement with both internal and external stakeholders throughout the process is key to understanding and anticipating the complexities ahead. From early development to clinical trial expertise along with commercial payer and provider solutions that provide effective market access strategies for new oncology therapies, we can fully support your assets throughout the world.
最能描述你的是什么?
实现更多有针对性的免疫肿瘤学疗法。
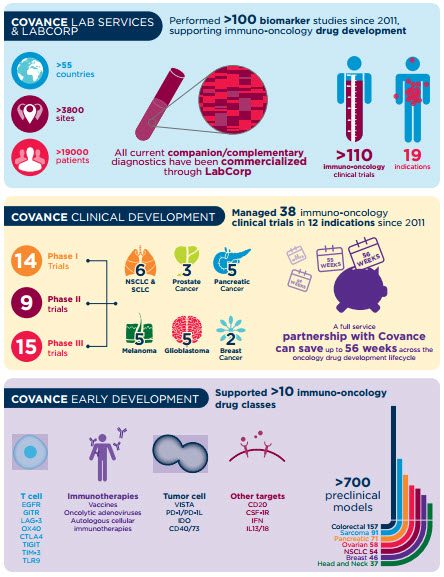
使收益大致ion medicine requires the use of novel methods to quickly test and target the right therapy to the right individual. At Covance, we have proven experience in immuno-oncology studies, including over 100 protocols in the past five years, performed in 58 countries at more than 2,860 sites with over 19,750 patients.
我们对免疫肿瘤学的承诺也通过我们的支持超过70%的支持也很明显FDA批准的伴侣诊断产品. As part of a recent pivotal Phase III registration trial,我们的中央实验室是PD-L1表达式的唯一提供者,导致了对Opdivo的新型FDA批准的诊断测试®(nivolumab). In addition, our best-in-class companion diagnostic capabilities supported the approval of TagrissoTM(Osimertinib)及其EGFR突变试验以及免疫疗法Keytruda®(pembrolizumab) and its PD-L1 companion diagnostic.
您可以利用我们在个性化药物开发和创新试验设计方面的深入体验,以开发和商业化您的药物和伴侣诊断。在一起,我们可以在现代肿瘤学中取得重大进展,并为患者带来创新的药物。
Improve your efficiencies with clinical informatics.
You can’t avoid risks in clinical trials, but you can control how you identify and manage those risks. TheXcellerate®临床Trial Optimization Platformoffers advanced analytical solutions to pinpoint risks at the study, site and operational level.
By leveraging advanced informatics, you’ll find and manage the right patients and sites to speed execution, address risks and gain more trial efficiency—all through one comprehensive platform.
一种加速进展的协同方法。
IO drug development has many uncertainties. That’s why collaboration is even more important to unite efforts and examine your compound from multiple perspectives. As your partner, we are uniquely positioned to engage, enable and facilitate key relationships and fully support the success of your innovative therapy.
Whether you needsolutions with biomarkers和伴侣诊断测定,proof of concept,临床试验或市场可行性和报销,我们的免疫肿瘤学经验和多学科专业知识的独特结合可以在您的旅程中产生差异