Early Phase Development Solutions
加速您的分子的成功,以方程方法给药,通过临时临时里程碑迅速推进您的分子,同时最大化资产的价值。在早期药物开发时间表上刮掉30%。
合伙。连续性。值。
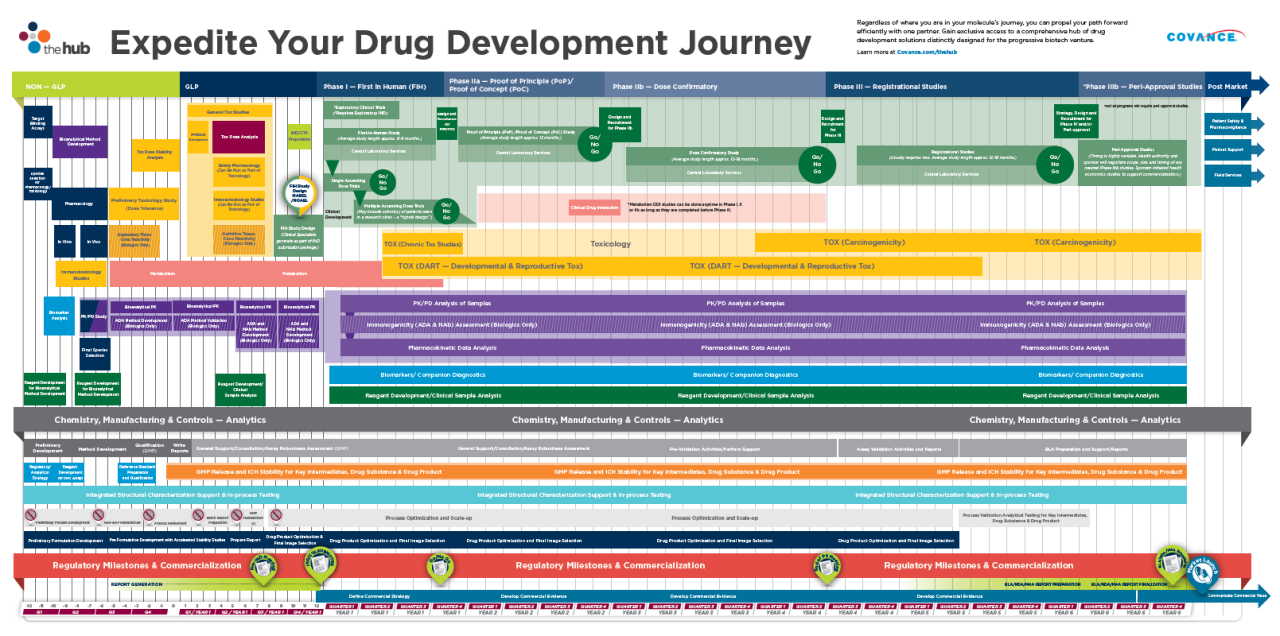
在早期药物开发时间表 - 从潜在候选人到概念证明 - 从概念证明 - 有了潜在的策略和计划。
Leverage a world-class drug development team that has led hundreds of small and large molecules in a plethora of therapeutic areas.
Work with one focused team, led by a drug development expert, that brings together scientific insight, regulatory guidance and program management for a smooth development of your molecule.
进入虚拟世界并体验早期发展解决方案。它可能是您今天花费的最有价值的3分钟。
Start with the end in mind to make smarter decisions—at every stage.
在药物开发过程中具有如此多的关键决策点,对早期药物开发效率的洞察力增加可以帮助您揭示潜在的风险和机会,并回答沿途所产生的众多问题:
- 如何加快我的计划,更容易回复投资者和利益相关者要求?
- 如何提前确定挑战,让我在没有失去时间或金钱的情况下进行调整?
- How do I demonstrate that my compound will be commercially attractive to licensors or partners?
- Is this the best regulatory strategy to mitigate risk?
- How do I align my nonclinical plan with my clinical endpoints and expedite my path into first-in-human?
From the beginning, you’ll prospectively get the right strategy for your unique program. With flexible solutions and continuous support to overcome uncertainties, you’ll reach your critical decision points, faster.
Your Journey is prospectively mapped out to optimize time and maximize value:
科学和运营连续性 - 从一开始。
Continuity is vital to the success of your molecule development. Early Phase Development Solutions provides you with direct access to a focused team of nonclinical, clinical and regulatory experts that will remain with you throughout your program. The result is a unified approach and consistent data package that sets you up for success.
Flexible solutions to match your unique needs.
Early Phase Development Solutions also brings a flexible, tailored approach to contracting, with options such as study-by-study or targeted milestones invoicing, all with guaranteed deliverables to meet your unique financial needs. It’s just another way you can maximize your asset’s value—and your bottom line.
How far you go is up to you
Whether you plan to complete an IND/CTA-enabling program or you need to gain the clinical insight that a first-in-human (FIH) or proof-of-concept (PoC) study can provide, you can enjoy the journey with a dedicated team and a singular, cohesive strategy that transitions seamlessly between nonclinical and clinical development.
Which candidate is best?
让集成解决方案迅速识别和develop your best lead candidate. From early characterization and formulation on development batches, to non-GLP screening for early identification of pharmacology, or toxicity-related issues—rest assured, you’ll move your best candidates forward.
IND/CTA-enabling nonclinical assessment
Take advantage of the vast knowledge of an expert team who manages drug development programs to support hundreds of regulatory submissions each year. With Early Phase Development Solutions, you seamlessly integrate the complete array of nonclinical services, including lead optimization, safety pharmacology, toxicology, pathology, bioanalytical, drug metabolism and pharmacokinetics, to assure successful design and conduct of your program—all the way through IND/CTA submission and into first in human clinical studies.
First-in-Human (FIH) Studies
利用早期开发解决方案,您可以从非纯粹的研究结果中受益于保留的知识,以更有效地将您的化合物移动到药物开发阶段。重点将是您FIH研究的两个关键方面:科学诚信和人类主体安全。由于早期研究仍然需要更复杂的研究,需要特殊人群,多个端点和自适应协议设计,您将通过35年以上的洞察力和行业领先的人类AME专业知识获得优势。
Proof of Concept (PoC)
等待直到您在设计概念证明之前有一个完整的数据包(POC)学习,可以浪费宝贵的时间。相反,您将通过并行处理可行性和现场评估,并纳入相关的生物标志物并利用适应性试验设计来享受这些更短的创新方法,科学苛刻的研究。通过对计划应用正确的医疗,科学和治疗专业资源和患者分层策略来增加临床投资回报率。
A Programmatic Approach – Why it Matters
如何在您的计划上刮起高达30%的人?这是为了简化您的旅程,取出空白,并重新思考风险管理。阅读更多“估计时间对药物发展计划,资产价值和金融公司绩效的影响” whitepaper. What’s more, we can sit down and do an economic valuator session – to estimate the likely time savings for your specific program.
Watch Peter Sausen, PhD explain how a programmatic approach to early development can shave up to 30% off your timelines to maximize your asset value at point of sale.
Then, join more than 225 biotech ventures that have experienced the real-world benefits of a programmatic approach over the course of the last three years.