Immuno-Oncology Clinical Trial Development
Accelerate your immuno-oncology studies by leveraging scientific and operational expertise.
Complex immuno-oncology drug development requires innovation, commitment and expertise
Develop your novel agents and companion diagnostics using comprehensive preclinical models and biomarker identification strategies
Optimize efficiency using advanced proprietary analytical platforms
Build lasting partnerships from proof of concept to market access with experienced investigators from Africa, the Americas, Asia-Pacific and the EU
Precision medicine. Advanced diagnostics.
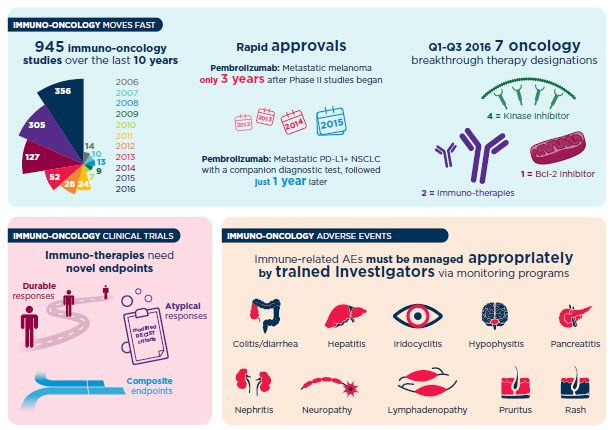
Our industry’s deeper understanding of the immune system’s involvement in cancer has uncovered new approaches in the treatment of cancer. You need to quickly screen and identify safe and efficacious molecules, both alone and in combination, to quickly advance to the next milestone. Leverage our deep science expertise and experience in precision medicine to support your testing needs and execute on innovative trial designs that allow for the success of your unique therapy.
是否帮助你找到最好的化合物为有限公司mbination therapy, identifying and stratifying biomarkers, developing companion diagnostics or revealing your market opportunities, our IO experience andxinyabo体育can deliver solutions from discovery through clinical trials and post-approval activities to advance your development at any stage.
Innovative informatics. Applied analytics.
Addressing the increased complexity of immuno-oncology drug development and the explosion of clinical and operational data requires new approaches to trial design and operational execution. Overcome inherent challenges by applying our multipronged approach that includes identification of premier sites, rapid startup and ongoing analysis of clinical data.
With proprietary tools like ourcentral laboratory database—with more than 40% of market data—along with LabCorp’s deep resource of patient aggregated and longitudinal data and theXcellerate®Clinical Trial Optimization Platform, you can leverage the latest analytical solutions to minimize risk, increase speed of execution and provide quality data to drive your decision making during your immuno-oncology clinical trials.
Enabling collaboration in an ever-changing environment.
Our industry’s ecosystem continues to shift. Rapidly advancing science, the nature of development and the commercialization process along with an increasing focus on patient centricity must be recognized to effectively facilitate progress.
Collaboration and engagement with both internal and external stakeholders throughout the process is key to understanding and anticipating the complexities ahead. From early development to clinical trial expertise along with commercial payer and provider solutions that provide effective market access strategies for new oncology therapies, we can fully support your assets throughout the world.
What best describes you?
Achieve more targeted immuno-oncology therapies.
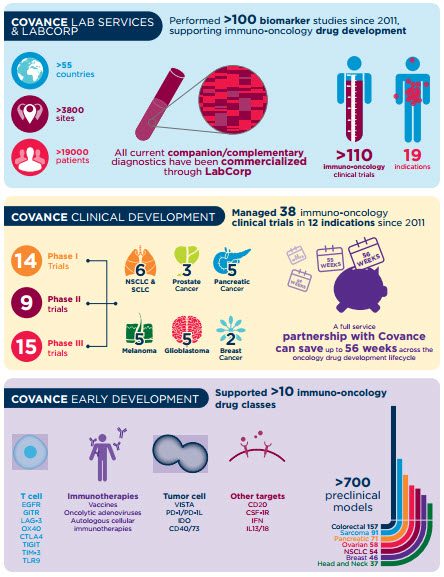
使收益大致ion medicine requires the use of novel methods to quickly test and target the right therapy to the right individual. At Covance, we have proven experience in immuno-oncology studies, including over 100 protocols in the past five years, performed in 58 countries at more than 2,860 sites with over 19,750 patients.
Our commitment to immuno-oncology is also evident through our support of more than 70% of allFDA-approved companion diagnostic products. As part of a recent pivotal Phase III registration trial,our central laboratorywas the sole provider of testing for PD-L1 expression, which led to a new FDA-approved diagnostic test for OPDIVO®(nivolumab). In addition, our best-in-class companion diagnostic capabilities supported the approval of TagrissoTM(osimertinib) and its EGFR mutation test along with the immunotherapy Keytruda®(pembrolizumab) and its PD-L1 companion diagnostic.
You can take advantage of our deep experience in personalized drug development and innovative trial design to develop and commercialize both your drug and companion diagnostics. Together, we can make significant advances in modern oncology and bring innovative medicines to patients.
Improve your efficiencies with clinical informatics.
You can’t avoid risks in clinical trials, but you can control how you identify and manage those risks. TheXcellerate®Clinical Trial Optimization Platformoffers advanced analytical solutions to pinpoint risks at the study, site and operational level.
By leveraging advanced informatics, you’ll find and manage the right patients and sites to speed execution, address risks and gain more trial efficiency—all through one comprehensive platform.
A collaborative approach to accelerate progress.
IO drug development has many uncertainties. That’s why collaboration is even more important to unite efforts and examine your compound from multiple perspectives. As your partner, we are uniquely positioned to engage, enable and facilitate key relationships and fully support the success of your innovative therapy.
Whether you needsolutions with biomarkersand companion diagnostic assays,proof of concept, clinical trials or market viability and reimbursement, our unique combination of immuno-oncology experience and multi-disciplinary expertise can make a difference in your journey