NCI-H2228 - 在非小细胞肺癌原位脑转移模型中使用DCE MRI
作者:
erin trachet,sr。科学顾问,肿瘤学/ sr.经理,提案开发
DATE:
September 2017
More than 90% of all pancreatic cancers are classified as ductal adenocarcinomas and, within the western-world, pancreatic cancer is the fourth leading cause of cancer related deaths. Prognosis with pancreatic cancer is extremely poor, with a 5-year relative survival rate of 5% and median survival of 3.5 months for patients with Stage III non-resectable tumors.1 Unfortunately, the incidence of pancreatic cancer has been on the rise while the 5-year survival rate has not changed. Surgical resection is the only potentially curative therapy, but only 10% of patients are diagnosed early enough for this to be an option and most who are eligible for surgery ultimately relapse. As with many other types of cancer, pancreatic cancer grows silently for years without any symptoms. In most cases diagnosis is not made until the cancer has grown outside of the pancreas to other proximal tissues and/or has metastasized. These patients are left with very few meaningful options. Therefore, effective novel therapies are sorely needed in treatment of pancreatic cancer.
在过去的15年中,诊断患有晚期胰腺癌的患者是吉西他滨(Gemzar®)作为标准的第一线治疗。yaboapp体育官网尿动突然地说,我们使用Gemcitabine作为我们的护理标准,为客户提供基准,以期望超越当前临床处理选项或与小说疗法结合;如靶向药物和免疫调节剂。
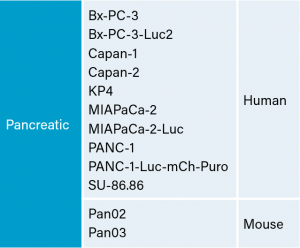
临床前癌症研究界有几种人和鼠胰腺细胞系可帮助开发新的疗法。yaboapp体育官网Covance有一个大面板的胰线,准备测试(见表1)。我们已经优化并表征了几种这些模型的皮下(SC)生长,并评估了对吉西他滨治疗的反应。
PANC-1
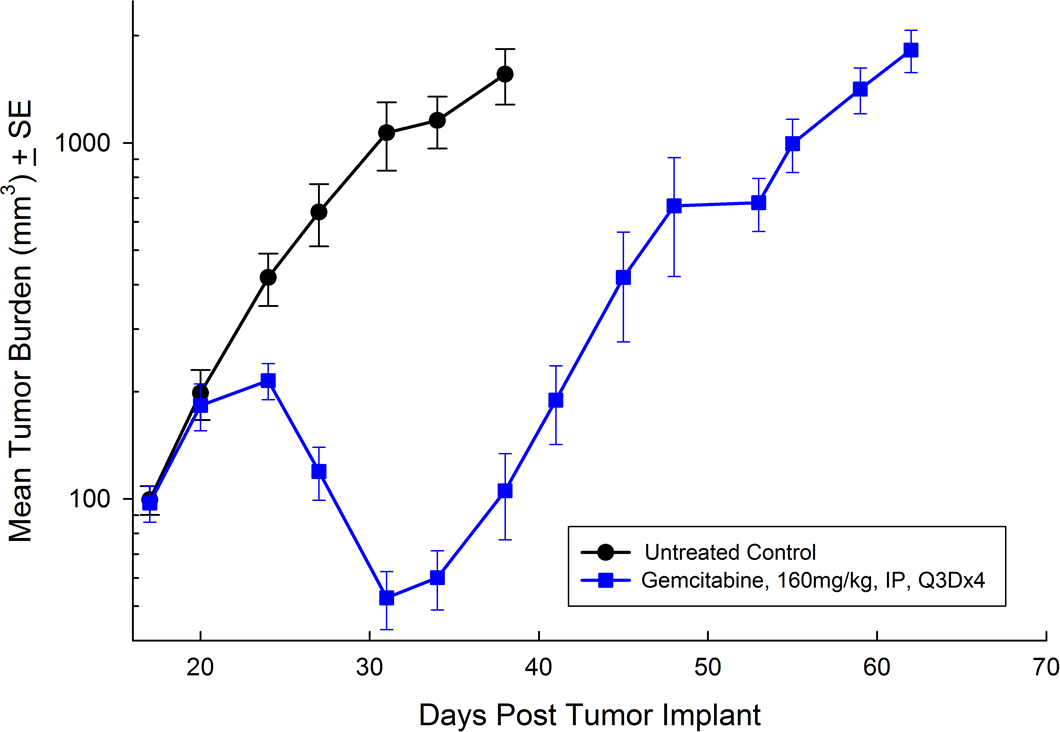
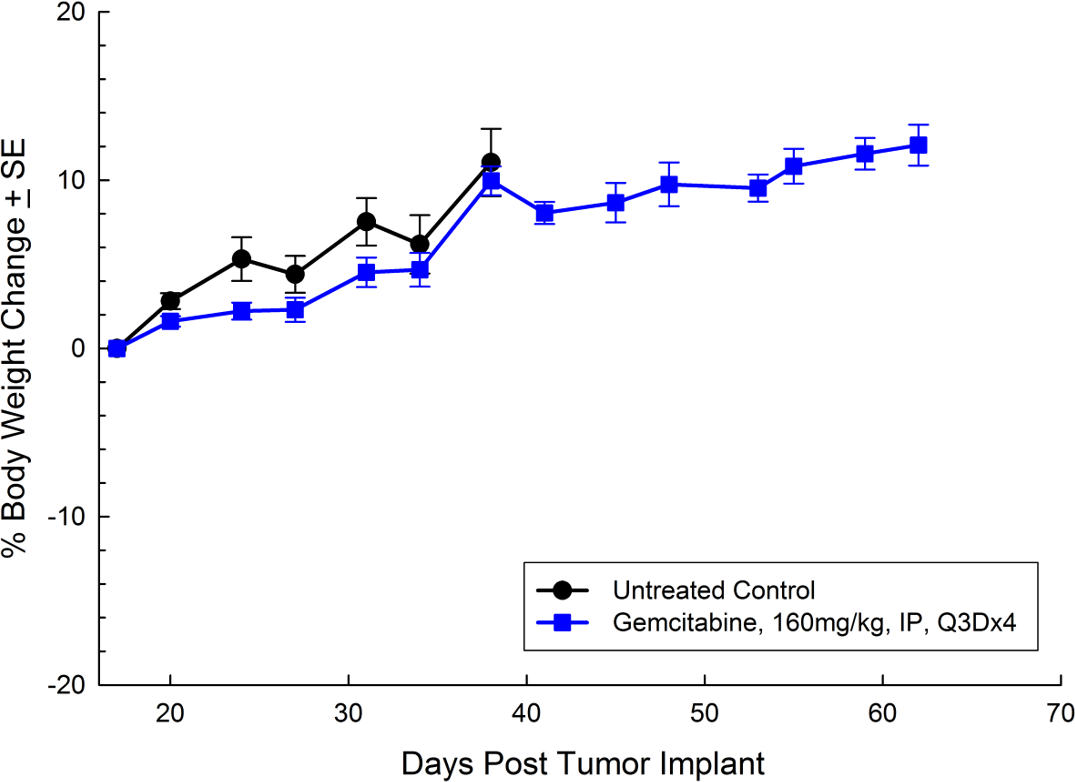
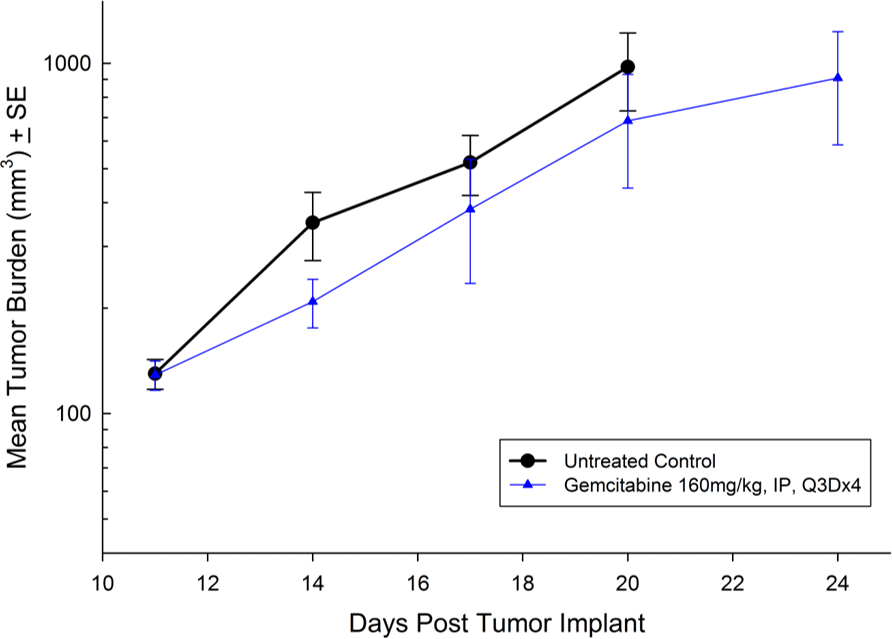
Panc-1被孤立于一个56岁的白种人男子,胰腺导管腺癌。皮下肿瘤生长可靠且一致,肿瘤体积每5天加倍,通常达到评价大小(〜750mm3.)在植入后大约29天。用吉西他滨(160mg / kg)治疗耐受良好的耐受性,并产生统计学上显着的肿瘤生长延迟,但不是疗效(见图1和2)。
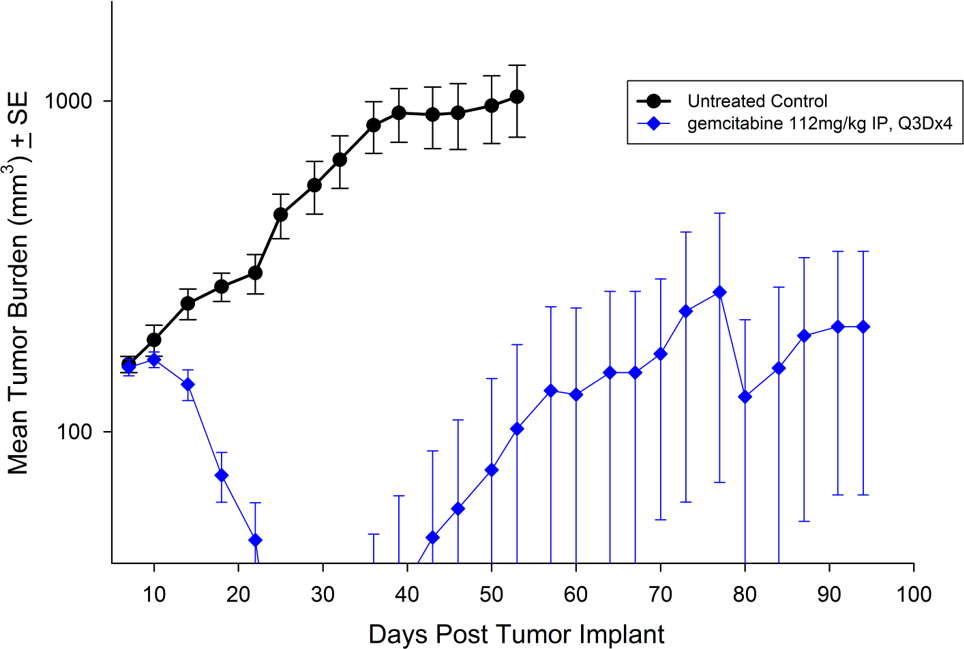
Capan-2
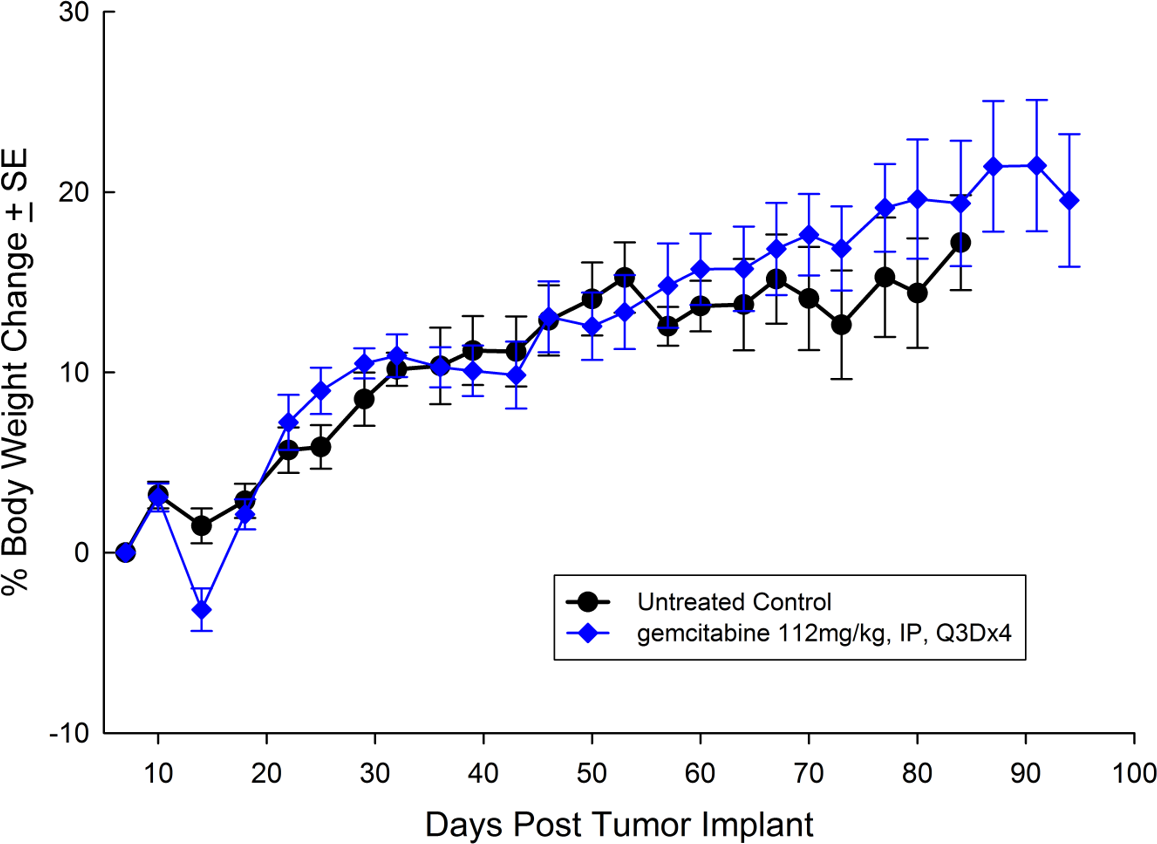
Capan-2, isolated from a 56-year-old Caucasian man with pancreatic ductal adenocarcinoma has subcutaneous tumor growth that is a bit slower than PANC-1 with a tumor volume doubling time of about 12 days and the time to reach evaluation size (750mm3.) of about 36 days post implant. Treatment with gemcitabine (112mg/kg) is well tolerated and produces statistically significant tumor growth delay, with a large number of tumor free survivors (see Figures 3 and 4).
Bx-PC-3
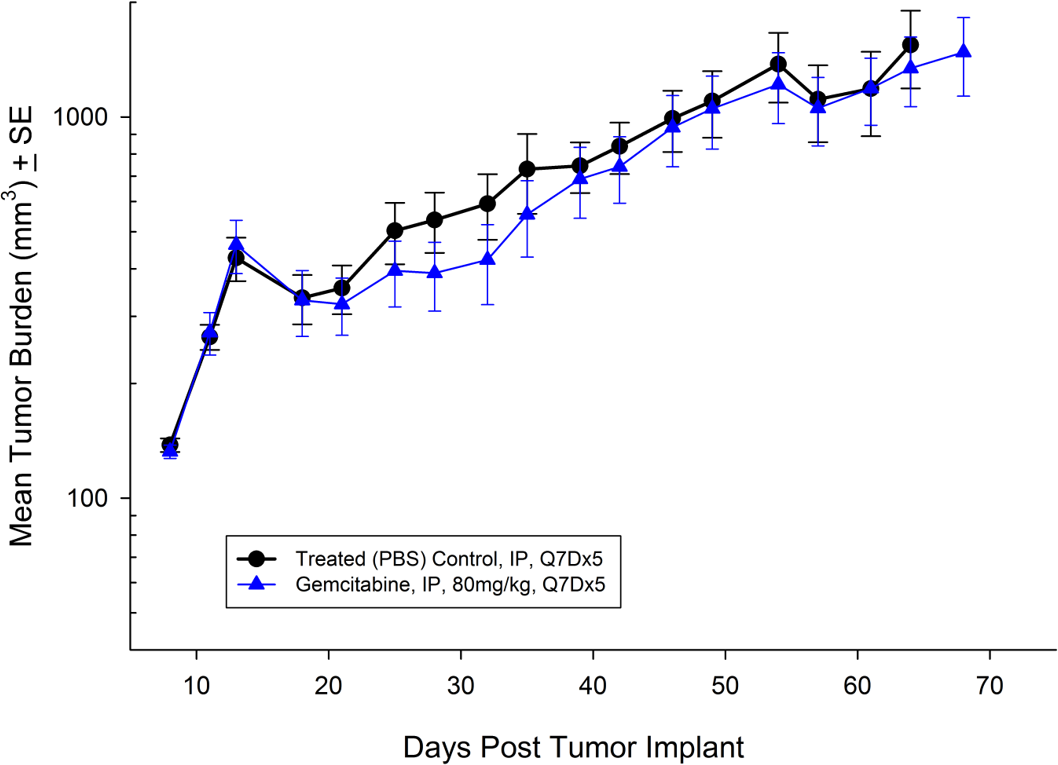
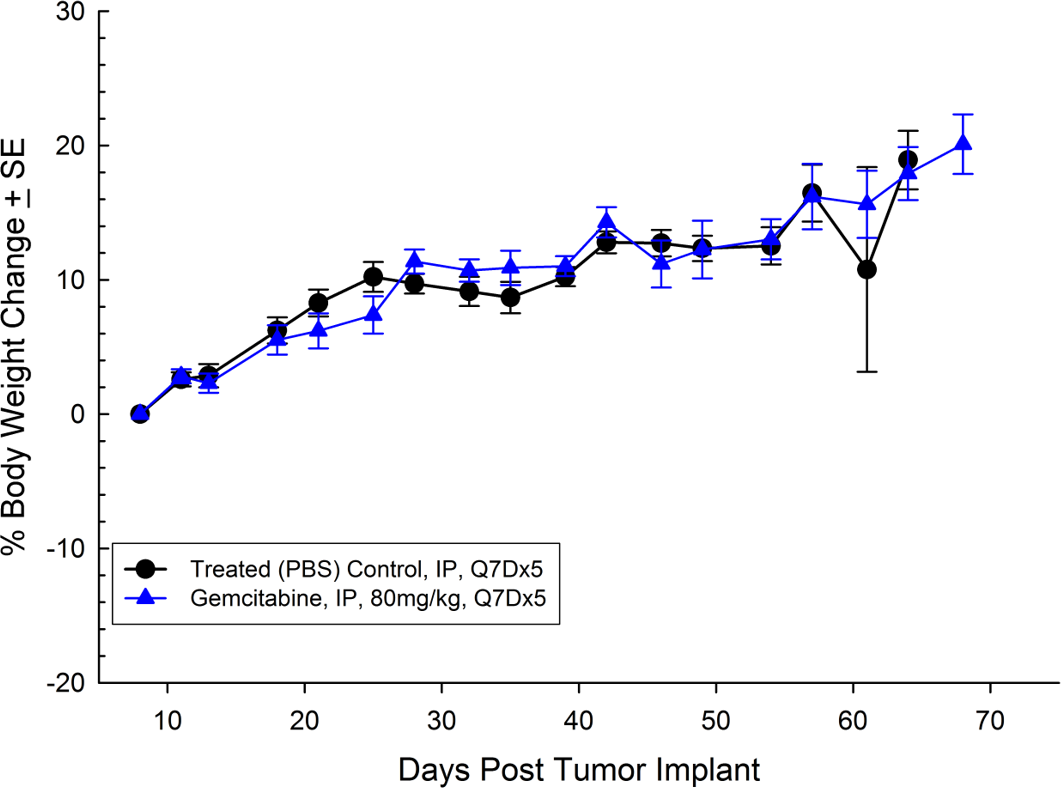
BX-PC-3从一个61岁的白种人女性中分离出胰腺癌。皮下肿瘤生长导致肿瘤体积倍增时间为约16天,达到评价大小的时间(750mm3.) of about 38 days post implant. Treatment with gemcitabine (80mg/kg) is well tolerated but fails to produce statistically significant tumor growth delay (see Figures 5 and 6).
Miapaca-2
Miapaca-2was isolated from a 65-year-old Caucasian man with pancreatic carcinoma. This model demonstrates very aggressive subcutaneous tumor growth with a tumor volume doubling time of about 3 days and the time to reach evaluation size (750mm3.植入后约21天。用吉西他滨治疗(160mg / kg)耐受良好的耐受性,并产生统计学上显着的肿瘤生长延迟,在该研究中观察到一种肿瘤游离幸存者(见图7和8)。
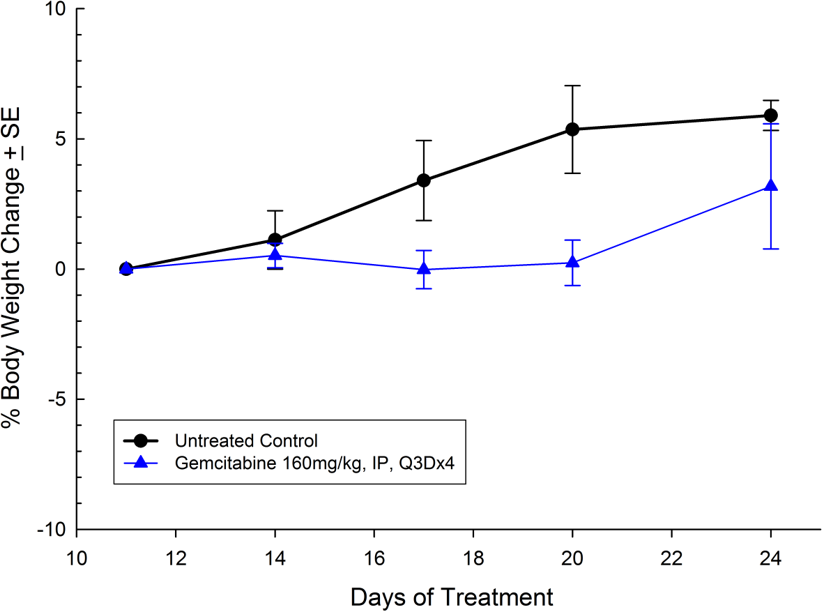
As in the clinical response to standard of care, treatment response preclinically is highly dependent on the tumor line being used. There are many factors that can contribute to the responsiveness or lack thereof to gemcitabine treatment. However, looking across several different tumor lines within the same indication can provide key information about how effective a new therapy may be in the clinic.
请联系我们if you are interested in discussing any of our pancreatic models.
1Siegel R. L., Miller K. D. & Jemal A. Cancer statistics, 2015. CA Cancer J Clin 65, 5–29 (2015). [PubMed]
Note: Studies were performed in accordance with applicable animal welfare regulations in an AAALAC-accredited facility