Companion Diagnostics
Parallel development of a companion diagnostic with a novel therapeutic is challenging — but the value of proving your treatment’s efficacy with targeted tests or biomarkers has never been greater. Precision medicine demands patient stratification – identifying target patients who are more likely to benefit from therapy; companion diagnostics also enable reduced development time and Rx development costs, can result in more favorable reimbursement and can illustrate comparative effectiveness.
A new option — called a complementary diagnostic — provides additional information about how a drug might be used, but is distinct from companion diagnostics. We supported the development of the first two FDA-approved complementary diagnostics, and can help you weigh your strategic options.
Learn how we invest in the future of precision medicine
Your therapeutics and companion diagnostics — in perfect harmony.
Covance is uniquely equipped to support your development of a personalized medicine with proven pathways to success.
Choose your pathway with anin vitrodiagnostic (IVD) or laboratory developed test (LDT)
Access diverse biomarker expertise from development to validation
Receive informed regulatory guidance along with a market access strategy
A balance of breadth and depth. Relevant experience across therapeutic areas and platforms
无情的一致性。全球统一临床试验的结果
Unique insights. Informed regulatory guidance and support
Commercialization solutions. A partner that can help you develop and implement your CDx market access strategy and launch
A balance of breadth and depth. Relevant experience across therapeutic areas and platforms
无情的一致性。全球统一临床试验的结果
Unique insights. Informed regulatory guidance and support
Commercialization solutions. A partner that can help you develop and implement your CDx market access strategy and launch
一起,Covance和Labcorp具有成功开发和商业化CDX的工具和跟踪记录。
Your therapeutic needs a companion; you need a resourceful partner.
伴侣诊断(CDX)可以将个性化药物的承诺转化为现实。我们的医疗和科学主题专家团队跨越所有治疗领域,您将协调您的RX和DX发展目标。与您的治疗性批准同时,我们将帮助您的CDX达到市场 - 更快。
The power of choice: in vitro testing or laboratory developed test.
Your best path to CDx commercialization may not involve a traditionally distributedin vitrodiagnostic (IVD) kit. Projects involving orphan drugs, compressed timelines or operational complexities may thrive with an FDA-approved, laboratory-based assay. You’ll minimize upfront investment and mitigate potential risks associated with a widely distributed IVD kit. As your partner, we’ll develop and validate your test, lead the regulatory submission and offer immediate global access to get your test to market quickly.
Whether pursuing a laboratory developed test (LDT) or an IVD, you need expert guidance to unite your CDx and therapeutic development and extract the most value from your trials. With more than 25 years of clinical trial experience and 80+ diagnostics delivered to market, we possess the technical arsenal, strategic agility and scientific insights that can help you determine the best approach to commercialization.
Global reach and consistency—driving your companion diagnostic validation.
The targeted nature of CDx development requires assays to be designed and validated across a spectrum of patient populations. You can capitalize on our global coverage, distribution networks and standardization efficiencies within our harmonized central labs. And, with high-caliber investigator sites worldwide, you get quality, combinable data every time.
Your possibilities. Our companion diagnostics solutions.
More than a checklist of relevant capabilities, our CDx services work in concert to support and accelerate all aspects of your co-development efforts. There is no substitute for experience: we have supported 19 of the ~25 FDA-cleared or approved companion diagnostic devices—including those for HER-2, KRAS, EGFR, BRAF and ALK. Unify your CDx pursuits with a single strategic partner, including:
- Biomarker identification and development
- Assay feasibility, proof of concept and validation
- Global clinical trial testing
- 设备制造和商业化的伙伴关系
- Premarket Approval (PMA) application development and submission
- Market access strategies, launch support and commercialization guidance
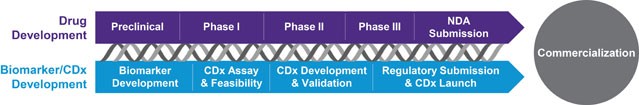
With experience that spans companion diagnostics and complementary diagnostics for oncology and immuno-oncology, CNS, infectious disease and inflammation, we can offer scientifically adept and focused expertise to drive your CDx success.

