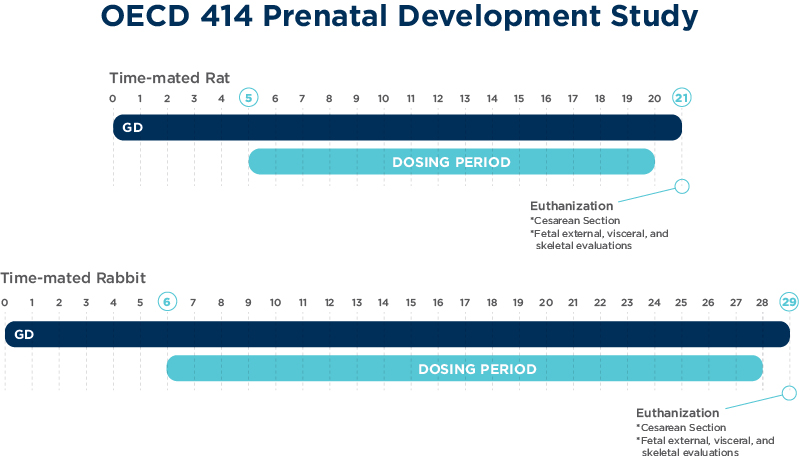
OECD 414: Prenatal development toxicity study
The objectives of the prenatal development toxicity study are to detect adverse effects on the pregnant female and development of the embryo and fetus consequent to exposure of the female from implantation to the day prior to parturition.
Aims of this study design are to determine whether enhanced toxicity occurs relative to non-pregnant females, and effects on embryo-fetal survival, fetal weight and fetal development.
Studies are able to be conducted in rodents and rabbits primarily via oral gavage route of administration.
This design can also be conducted as a preliminary dose-range finding study with a smaller sample size and fewer fetal evaluations in order to determine appropriate dose levels for the definitive study.