The NIH defines precision medicine as “an emerging approach for disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle for each person1。” In cancer patients, we can rephrase the definition to “through detailed understanding of a cancer’s biology, providing the right drug, for the right patient, at the right time.”
In order to identify the correct drug, biomarkers are used to identify patients that can be treated with the appropriate therapy for their cancer. The FDA defines biomarkers as “a defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions2。” Great strides have been made in the discovery and validation of biomarkers in drug development.
临床试验中最常见的两种生物标志物是预测性和药物动力学生物标志物,对药物开发过程至关重要。预测生物标志物是那些识别最有可能应对或抵抗药物的患者的生物标志物。预测敏感生物标志物的一个例子是使用免疫组织化学(IHC)的HER2乳腺或胃癌的染色。乳腺肿瘤的患者,将被认为用Herceptin®治疗来考虑HER2蛋白的乳腺肿瘤(分数为2+或3+)。抗性生物标志物也很重要。例如,如果人的肿瘤组织具有KRAS突变,Panitumumab(结直肠癌中批准的EGFR抗体)是禁忌的3。这些类型的生物标志物在早期的发展阶段,毒品公司最常选择。使用动物和细胞模型来识别和最初表征这些生物标志物的数百万美元。随后,如果它们在临床试验中验证,这些生物标志物可能成为对发育中药物的伴侣诊断。这些生物标志物对药品公司非常有用,因为它们有助于通过保持高性化合物的安全性和疗效来发展患者的合适群体。
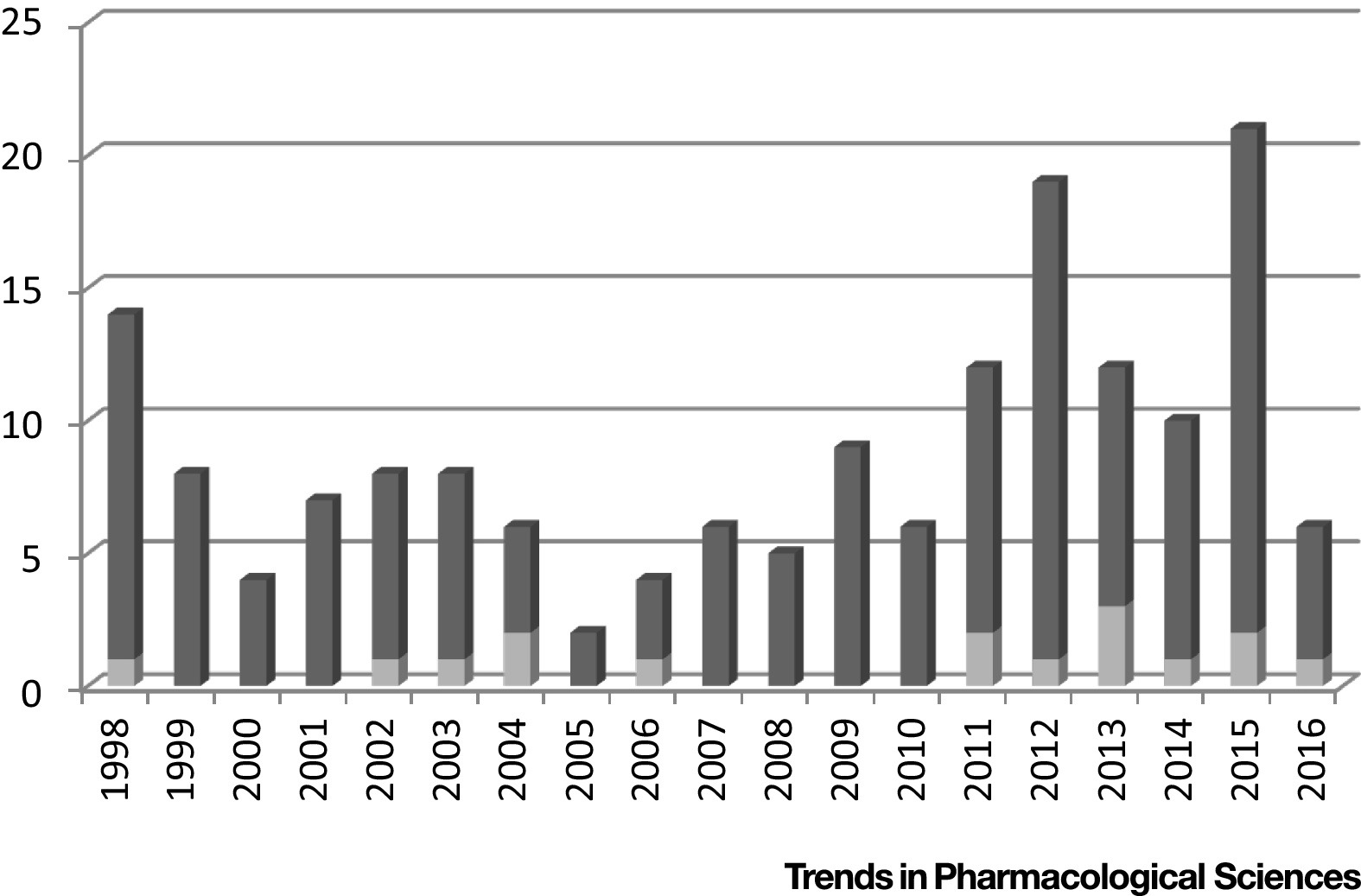
Finding predictive biomarkers that can be developed into companion diagnostics is important in precision medicine, but is not an easy task to accomplish. Two things need to happen simultaneously: the correct biomarker must be found and the associated drug must get approved. However, drug approval is not easy. Of all the drugs that enter clinical testing, only about 10 percent will receive approval from federal agencies. In addition, there are thousands of biomarkers that are tested, but only a few enter clinical trials4, and fewer become companion diagnostics. A total of only 32 companion diagnostics have been approved by the FDA for 16 drugs, or roughly only 9 percent of all approved drugs since 1988 have a companion diagnostic (figure 1)5。These numbers clearly indicate the difficulty of finding and validating biomarkers that result in a companion diagnostic. Yet, finding them can help save hundreds of millions of dollars for a company that is developing a drug.
Figure 1.
药效动物生物标志物是在复合处理后改变的标记物。它们很重要,因为它们提供了分子的作用机制(MOA)的线索。药效学生物标志物在人类测试的早期阶段非常有用,因为它们可以验证药物的拟议活性途径。通过观察药物抑制的途径或通过在测试中观察到的模型中观察到的二次效果中,这些通常在临床前发展中被发现。虽然药效动动力学生物标志物大量用于临床前和I期临床试验,但它们的使用在药物开发过程的后期阶段急剧下降。这些生物标志物往往不被用作伴侣诊断。
将精密药物应用于免疫肿瘤学(IO)不是过去使用的方法的直接应用。由于患者的免疫系统被治疗以对癌症作出行动以来,需要考虑几个因素。因此,需要检查免疫系统和肿瘤。当制定药效学生物标志物时,研究人员可以看待特定免疫细胞的免疫细胞,增殖或激活状态的整体数量的变化,作为药物活性的指标。一个例子是用Yervoy®,CTLA-4抑制剂治疗的患者的外周血中存在所有淋巴细胞计数。发现随着抑制剂的增加治疗的患者具有较高水平的ALC,表明该药具有下降调节调节T细胞活性的所需效果,从而观察循环中更多的淋巴细胞(图2)7。,)
Figure 2.
此外,可以通过测序与肿瘤相关的那些细胞的T细胞受体(TCR)来查看T细胞的总数和克隆性8。The clonality of the T cells associated with the tumor is an indicator of the immune response to the tumor. In addition to providing clues to the exact mechanism of action, these biomarkers can also point to the therapeutic choice for the appropriate IO drugs. For example, if a patient shows that the immune system can recognize his or her own tumor, then using a check point inhibitor, like PD-1 or CTLA-4 inhibitors may be an appropriate course to follow. On the other hand, if the immune system has not recognized the tumor, then stimulation with a vaccine may be the appropriate therapy to follow. Biomarkers can help figure out the mechanism used by the immune system to eradicate the tumor and give clues to what type of medication will be more appropriate for each patient.
Predictive biomarkers are also found in IO, especially those associated with the tumor itself. Several methods have been used to discover them: pathological staining to look at the type of cells present in the tumor, including immune cells; comparing the ratio of effector T cells vs T regulatory cells; and the presence of immune cells in the leading part as compared to those outside of the tumor. Interestingly, one biomarker has already been developed as a companion diagnostic in IO, PD-L1 IHC. Patients that are positive for this marker can be treated with Keytruda (pembrolizumab) aPD-1 inhibitor(figure 3)9, thus providing the first example that precision medicine can help increase the response in immunotherapy. One word of caution, while the selection of patients increases the overall response rate, there are about 10-25 percent of patients that are low expressers or negative for this test and are able to respond to the drug (compare green and orange lines with the black line of patients with higher than 50 percent of staining levels). More work is needed with this drug to increase patient response rates.
The ongoing advances in cancer immunotherapy together with precision medicine may promise a bright future for patients. Immunotherapy is starting to prolong the life of some patients up to 10 years10。正在开发新药来治疗免疫系统的不同部位,从新型检查点抑制剂到疫苗和新抗原。通过合并这两种方法,免疫疗法和精确药物,目标是增加对药物响应患者的患者的百分比,希望更加持久和持久的反应。虽然乐观,但是设想癌症可以降低到慢性条件的世界并不是不合理的。
SusoPlatero,Ph.D., is Executive Director,Precision MedicineOncology Leader, Clinical Development Services,Covance, Inc.
1https://www.nih.gov/precision-medicine-initiative-cohort-program
3Amado et al.,J Clin Oncol。,2008年
4Kern, SCancer Research, 2012
5Dracopoli et al,Trends in Pharmacological Sciences2017; 38: 41-54
6Wolchok等人,TheLancet Oncology2010;11:问题2,155-64
7Robins等人。,科学翻译医学, 2013年12月
8Garon E et al.,n Engl J Med2015;372:2018-2028
9Schadendorf et al.,JCOdoi:10.1200/JCO.2014.56.2736