Date:May 2021
Contactthe preclinical oncology scientific team to learn more.
Covance by Labcorp Preclinical Oncology offers the new mouse Expanded CompMyeloid™ panel, which provides deep immunophenotypic analysis into myeloid subsets known to regulate the tumor immune response. The panel addresses the growing need to understand how immunotherapy modulates pro-tumor and anti-tumor activity in the myeloid compartment of the tumor microenvironment. In this Tech Spotlight, we demonstrate the power of this 18-color panel that provides a deep dive into myeloid subset immunophenotyping and functional characterization.
扩展的CompMyeloid™面板在我们的CompMyeloid™面板上构建,其提供了五个骨髓亚群的一般测量,可调节肿瘤中的免疫力。改进的面板添加DC1(类型1)和DC2(型)树突状细胞描绘,加上六种另外的标记,用于肿瘤相关的巨噬细胞(TAM),髓样衍生的抑制细胞(MDSC)和树突细胞亚群的功能表征。表1描述了扩展的Compmyeloid TM面板的组分,图1说明了使用鼠HEPA 1-6肝细胞癌模型的髓样子描绘的门控策略。
表1:扩展Compmyeloid™面板抗体和其实用程序的描述
Antibody/Dye |
描述 |
CD45 |
平移免疫细胞标记 |
CD11b |
Pan-myeloid谱系标记/ DC2子集标记 |
CD103 |
DC1 subset marker |
Ly-6G |
粒细胞-MDSC / Neutrophil标记 |
Ly-6C |
Monocytic-MDSC/Monocyte marker |
CD11c |
树突状细胞标记 |
CD24 |
Dendritic cell delineation |
F4/80 |
Pan-macrophage marker |
MHC Class II |
M1 macrophage & dendritic cell marker |
CD206 |
M2 macrophage marker |
XCR1 |
交叉呈递/迁移树突细胞标记 |
CD80. |
Maturation marker |
CD86. |
Maturation marker |
ARG1 |
骨髓功能标记 |
iNOS |
骨髓功能标记 |
PD-L1 |
T细胞抑制信号蛋白 |
CD19 |
B cell marker |
Viability Dye |
Dead cell exclusion |
The Expanded CompMyeloid™ panel provides immunophenotypic analysis of 7 distinct myeloid subsets including G-MDSC/neutrophils, M-MDSC/monocyte subsets, M1 and M2 Macrophages, and DC1, DC2 and XCR1+ dendritic cell (DC) subsets. The analysis of 5 myeloid functional markers to profile dendritic cell, macrophage, and MDSC phenotypes is also provided.
|
|
Expanded CompMyeloid™ Panel Gating Strategy
在Labcorp临床前肿瘤学免疫脑型面板与所有Covance对齐,分析以死区排除和yaboapp体育官网随后的CD45开始+immune cell delineation. Gated on total CD11b+cells, Figure 1A displays downstream MDSC and TAM analysis. Figure 1B demonstrates DC analysis that follows a meticulous gating strategy that first excludes macrophages, granulocytes, and B cells, which minimizes the risk that the DC measurement is compromised due to contamination by irrelevant and potentially autofluorescent cells. The exclusion gate then enables CD24 analysis (not shown) that helps delineate conventional DCs, which are generally classified by medium to high expression levels of CD11c and MHC class II. These conventional DCs include the CD103+DC1 subset and the CD11b+DC2子集,已被证明驱动CD8+and CD4+T cell anti-tumor responses respectively1。XCR1 expression has been documented by multiple groups as a marker for DCs with cross-presenting activity, a phenotype required for DC-mediated activation of CD8+T细胞2。
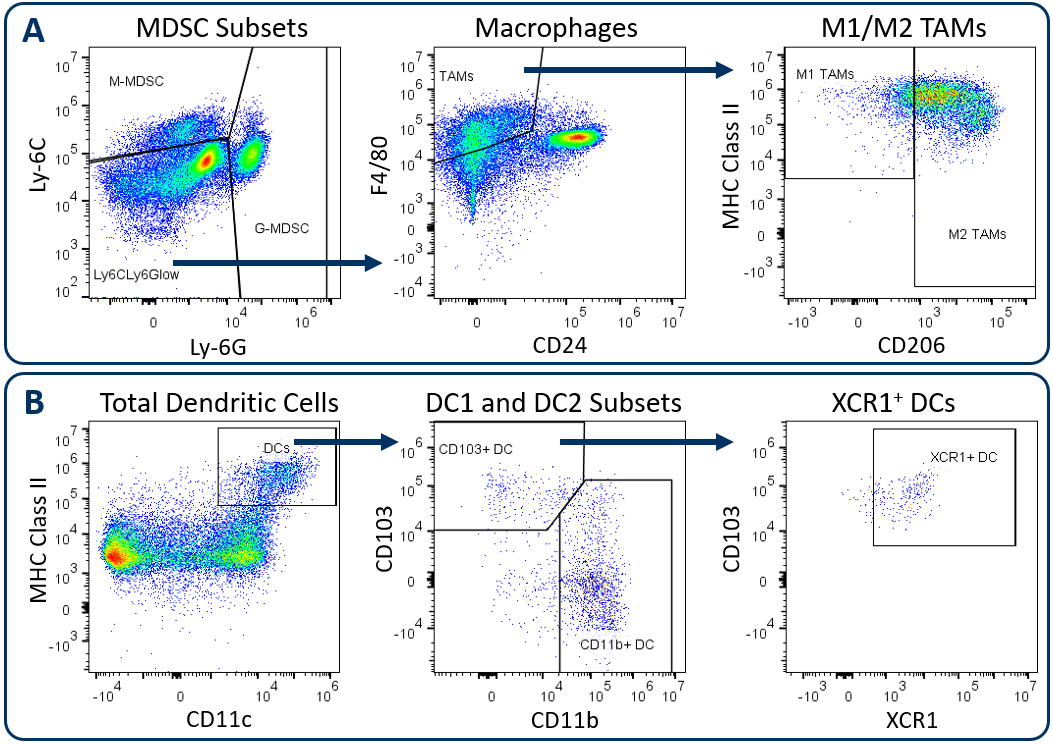
Figure. 1. Analysis of tumor-infiltrating myeloid subsets using the Expanded CompMyeloid™ panel. Hepa 1-6 tumors were harvested from C57BL/6 mice. (A) Starting from a CD11b+cell gate, G-MDSC and M-MDSC subsets are measured and the remaining events are delineated into M1 and M2 TAMs, (B) Starting from a macrophage/MDSC exclusion gate, DC1 and DC2 subsets, and an XCR1+DC fraction were measured.
All animal work is approved by the site Institutional Animal Care and Use Committee and was performed in conformance with theGuide for the Care and Use of Laboratory Animals在AAALAC认可的计划中。所有研究都预先确定了人文的安乐死标准。
iNOS and Arginase 1 Analysis in MDSC and TAM Subsets
Myeloid subsets impart activity in the tumor microenvironment that have both pro-tumor and anti-tumor effects. The Expanded CompMyeloid™ panel provides an analysis of iNOS and Arginase 1 (ARG1); biomarkers that are examples of the duality of myeloid function. iNOS and ARG1 are expressed in both MDSC and TAM subsets in the tumor3。In MDSCs, both iNOS and ARG1 mediate an immunosuppressive effect by competing for the substrate L-arginine that fuels T cell proliferative and anti-tumor responses. Tumors that have high iNOS and ARG1 expression can cause an L-arginine deprived environment that leads to poor T cell activation. Thus, therapies that reduce the expression of iNOS and ARG1 in MDSCs have the potential to promote T cell activation and improve clinical outcome. Notably, iNOS expression can also inhibit tumor growth in a T cell-independent manner, as the nitric oxide gene product can have direct cytotoxic effects on tumor cells to inhibit disease progression. iNOS expressed by M1 polarized macrophages can induce growth arrest as well as induce apoptotic cell death in different models4,5。Conversely ARG1 expression by M2 polarized macrophages in the tumor microenvironment suppress T cell responses in a manner mechanistically similar to MDSCs.
图2演示了使用皮下HEPA 1-6肝细胞癌模型的膨胀Compmyeloid™面板如何在骨髓子集中检查INOS和Arg1。我们使用该模型,因为通过抗PD-1治疗检查点抑制抑制肿瘤生长(未示出),从而提供机会检查米利电隔室如何在检查点封锁期间如何响应。与对照动物相比,抗PD-1治疗增加了INOS和ARG1在肿瘤浸润的粒细胞(G-)和单核细胞(M-)MDSC亚群中的表达(图2A)。相比之下,总TAM的分析表明InOS表达的增加与Arg1表达的小而显着降低(图2b)。值得注意的是,在INOS阳性TAM中,似乎具有双相酰基INOS表达谱的细胞。此外,INOS中MHC II类的表达更高highcell fraction indicating an enhanced capacity for antigen presentation and T cell activation. Being that iNOS and ARG1 are markers for M1 and M2 polarization respectively, the data suggest that anti-PD-1 treatment shifts the TAM balance toward M1 polarization and anti-tumor phenotype. The data are also a reminder that the tumor immune response is dynamic, and that immunotherapy can trigger multi-layered effects that push and pull between pro-tumor and anti-tumor activity.
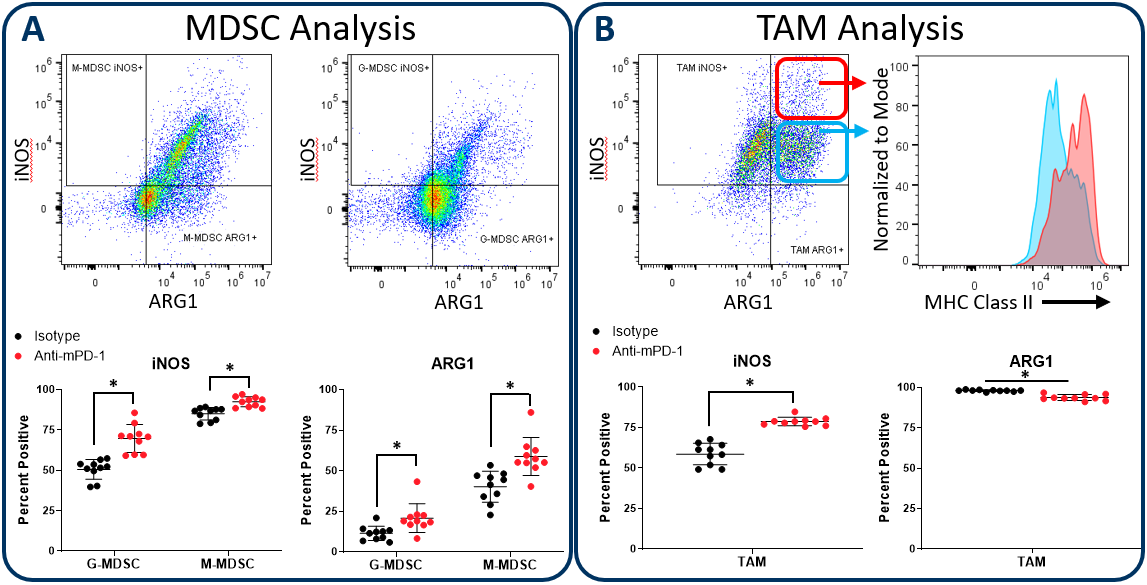
图2。伊诺和精氨酸酶1在MDSC和分析TAM subsets. The Expanded CompMyeloid™ panel measures biomarkers for myeloid functionality. In this data, Hepa 1-6 tumors were harvested from anti-PD-1 and isotype control antibody treated mice. MDSCs (A) and TAMs (B) were then delineated and analyzed for iNOS and ARG1 expression. TAMs differentially expressed relatively high (red circle enclosed) and low (blue circle enclosed) levels of iNOS expression, which directly correlated with MHC class II expression in these cell fractions, which is consistent with M1 polarization (red and green gates are representative examples and were not in this case used to generate the MHC class II measurement shown). * Student’s T Test (p<0.05).
CD80.and CD86 Maturation Marker Expression on Conventional DC Subsets
在肿瘤微环境中的工作中的常规树突细胞可以主要分为称为DC1和DC2的两个子集,其分别通过CD103 / XCR1和CD11b的表达鉴定。两个子集可以激活T细胞。但是,已显示DC1细胞有利于CD8+T cell activation due to an enhanced capacity for cross-presentation of tumor-associated antigens in the draining lymph nodes, a process that is regulated by XCR1 expression6。在上述HEPA 1-6研究中,扩展的Compmyeloid TM组用于测量CD80和CD86,其是在抗原呈递细胞上表达的共和素分子,并通过细胞接触依赖机构最大的T细胞活化所需的最大T细胞活化7。Figure 3 illustrates that both CD80 and CD86 are detectable on Hepa 1-6 tumor-derived dendritic cells. Furthermore, treatment with anti-PD-1 increased the expression levels of CD86 on the DC1 subsets compared to the isotype control group. The data suggest that enhanced T cell activation may be triggered by anti-PD-1 antibody treatment that is in part due to increased expression of CD86 on DC1 cells to enhance costimulatory activity in this subset.
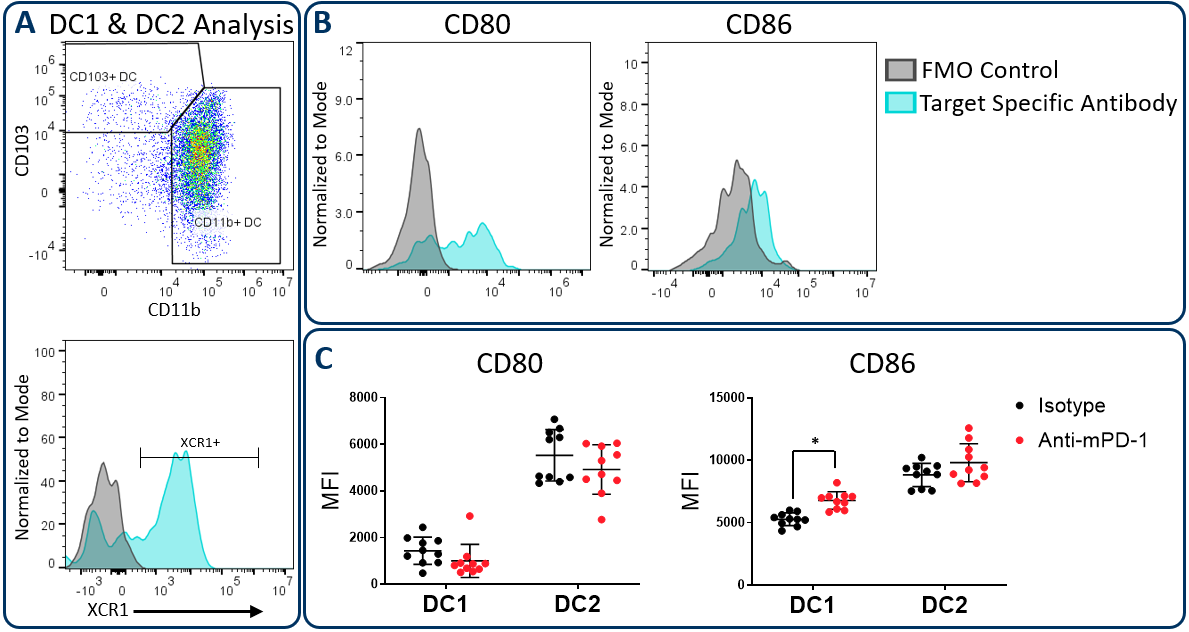
Figure 3. CD80 and CD86 maturation marker expression on DC subsets. The Expanded CompMyeloid™ panel was used to measure CD80 and CD86 in Hepa 1-6 tumor-derived cells. (A) Total DCs were first delineated and then phenotyped for DC1 and DC2 subsets. The gating strategy for XCR1 measurement is also shown. (B) Histograms to demonstrate representative CD80 and CD86 signals compared to the fluorescence minus one (FMO) negative control in DC1 cells. (C) CD80 and CD86 was measured in DC1 and DC2 subsets from Hepa 1-6 tumor-derived cells. * Student’s T Test (p<0.05).
骨髓亚群和肿瘤细胞的PD-L1分析
The PD-1/PD-L1 axis is one of the most widely targeted pathways in cancer immunotherapy. PD-L1 can be upregulated on tumor cells as a mechanism to evade host protective immunity. In addition, PD-L1 expression has been documented on myeloid cells including macrophages, dendritic cells, and MDSCs to further suppress T cell activation8。In some cases, PD-L1 expression in these distinct cell types can have non-redundant roles9。Together, these observations underscore the importance of understanding how PD-L1 expression in the myeloid compartment of the tumor can be modulated by immunotherapy. Figure 4 demonstrates the capability of the Expanded CompMyeloid™ panel to measure PD-L1 on total TAM, DC, G-MDSC/M-MDSC subsets, as well as CD45-(non-immune) cells, which include tumor cells, and also vascular endothelial and other cells. As shown below, PD-L1 is detectable in all cell types examined from Hepa 1-6 tumor-derived cells. The data also shows that compared to control animals, anti-PD-1 therapy had no effect on the levels PD-L1 expression in these cells, suggesting PD-L1 is not modulated by checkpoint inhibition in this model.
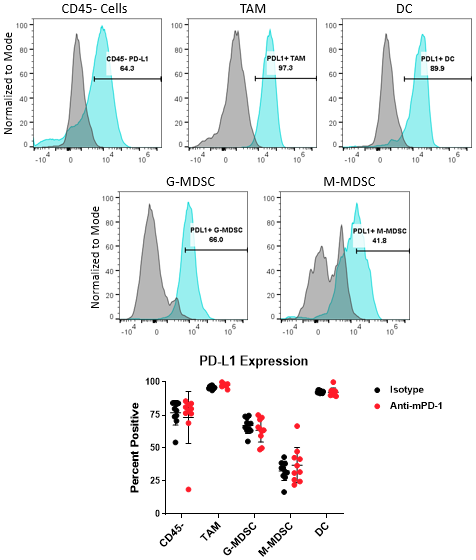
图4.骨髓亚胞胎和肿瘤细胞的PD-L1分析。Histograms demonstrate representative PD-L1 signals compared to the fluorescence minus one (FMO) negative controls in CD45-cells and myeloid subsets from Hepa 1-6 tumor-derived cells. The gate shown was used to quantify the percentage of cells that are positive for PD-L1 expression in the indicated subsets contained in the isotype control and anti-PD-1 antibody treated groups.
With the use of the Expanded CompMyeloid™ panel, the data in this Tech Spotlight presents evidence that checkpoint inhibition modulates the immune activity in multiple myeloid subsets in the murine Hepa 1-6 tumor model. Furthermore, the data provides additional support that anti-PD1 treatment exerts effects that are not limited only to T cell activity. To learn more about how the Expanded CompMyeloid™ panel can be incorporated into your preclinical research, contact the scientists at Covance by Labcorp.
再保险ferences
1加德纳,A.,&Ruffell,B。(2016)。树突细胞和癌症免疫。Trends in immunology,37(12), 855-865.
2Wylie,B.,Seppanen,E.,Xiao,K.,Zemek,R.,Zanker,D.,Prato,S.,...与Waithman,J.(2015)。迁移XCR1 + CD103和XCR1 + CD103 +树突细胞的皮肤黑素瘤抗原的交叉呈递。Oncoimmunology,4(8),E1019198。
3Kumar, V., Patel, S., Tcyganov, E., & Gabrilovich, D. I. (2016). The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends in immunology, 37(3), 208-220.
4Rahat, M. A., & Hemmerlein, B. (2013). Macrophage-tumor cell interactions regulate the function of nitric oxide. Frontiers in physiology, 4, 144.
5Vannini, F., Kashfi, K., & Nath, N. (2015). The dual role of iNOS in cancer. Redox biology, 6, 334-343.
6Wylie,B.,Seppanen,E.,Xiao,K.,Zemek,R.,Zanker,D.,Prato,S.,...与Waithman,J.(2015)。迁移XCR1 + CD103和XCR1 + CD103 +树突细胞的皮肤黑素瘤抗原的交叉呈递。Oncoimmunology,4(8),E1019198。
7Slavik, J. M., Hutchcroft, J. E., & Bierer, B. E. (1999). CD28/CTLA-4 and CD80/CD86 families. Immunologic research, 19(1), 1-24.
8Sun, C., Mezzadra, R., & Schumacher, T. N. (2018). Regulation and function of the PD-L1 checkpoint. Immunity, 48(3), 434-452.
9Oh, S. A., Wu, D. C., Cheung, J., Navarro, A., Xiong, H., Cubas, R., ... & Mellman, I. (2020). PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nature Cancer, 1(7), 681-691.
连接
Let's start a conversation
Contact Us