Using aCGMP.您的CRU的药房I药物制造的药物制造在质量和安全性,时间轴减少和成本效率方面的益处。
The regulatory environment continues to move toward requiring drug manufacturing at current good manufacturing practice (cGMP)-compliant pharmacies. This trend and other factors make it increasingly attractive to use cGMP compounding on-site at your CRU for early development. Let’s look atThree Big Benefits对于I阶段药物制造业:
- Quality and safety
- 时间轴减少
- 成本效益
行业趋势和监管变化
作为背景,让我们查看行业趋势,了解监管变化如何影响I阶段药物制造。近年来,灾难性事件在监管活动中推动了一个上涨。2011年,马萨诸塞州的复合药剂制作了污染的类固醇注射,用于疼痛管理。他们出现故障的复合实践导致了700例患有20个州的真菌脑膜炎,导致64例死亡。这种发生在药物复合行业中引起哗然,增加了为医生办公室,患者和临床试验提供产品的公司之间的恐惧。该活动导致FDA强烈监督,导致涉及个人的刑事指控。他们的评论表明,最大化的利润推动了这项业务,公司在该过程中牺牲了质量。
Since then, the Food and Drug Administration (FDA) has issued 130+ Form 483s (investigator observations that conditions or practices indicate a possible violation of FDA requirements) to pharmacy compounders across the U.S. These warnings focused on training, facilities, processes and quality expected at GMP facilities. Investigations led to legislation, namely the Compounding Quality Act, Title I of the 2013药物质量和安全法,改变了药物复合行业。这种联邦监督复合转化为复合无菌药品的药房的要求。
In short, we see that the inevitable move to cGMP is a result of increasing FDA oversight to protect the public. The investment required for organizations makes sense from a quality standpoint – to ensure that products produced at CRUs meet cGMP requirements.
利益#1 - 质量和安全
PREMIER对CGMP的利益结合了质量和安全性。质量是临床药物开发和制造的任何部分固有的,而I阶段的试验都是关于安全的。阶段我是我们寻找对药物的初始安全响应的地方,因为它是从临床前进程到临床世界的入学点。yaboapp体育官网
Why use a cGMP pharmacy located on-site at your CRU? The real distinction between a cGMP pharmacy and a traditional compounding pharmacy is that a cGMP pharmacy offers a quality management system (QMS), not just pharmacist oversight. A Phase I CRU having a cGMP pharmacy on-site can meet quality specifications and also leverage complete process information related to chemistry, manufacturing and controls (CMC) ─ including sterilization and containment systems ─ for use in later trial stages and investigational new drug (IND) amendments.
阶段I Regulatory Oversight
虽然监管监督是一个关键问题,但FDA审查所需的信息量,以确保质量在临床药物开发的每一阶段都有所不同。在我的发展中,我们已经知道了几个事实:
- 提供药物和产品纯度型材。
- The drug is used for a short duration.
- There are a limited number of patients involved.
- There is complete control over making and administering the drug.
因此,FDA意识到阶段的药物制造不应要求在后期阶段监督所需的相同级别的信息。目前的监管和指导要求质量水平符合标准的制造实践,指定:
- Innovation should not be impeded in early clinical development stages due to risk and costs to sponsors in early drug development.
- At each phase FDA rigor will determine the amount of information regarding
- CGMP.principles in ID Testing, Quality, Purity and Strength of the investigational drug product (IDP). 21 CFR 312.23(a)(7)(i).
While this gives some flexibility to organizations manufacturing drugs for Phase I trials, the guidance document does say that the FDA will exercise oversight of the study drug under general cGMP authority. In July of 2008, the FDA released a guidance document for investigational drugs detailing the exemption of drugs manufactured to meet cGMP for Phase I trials. This document is helpful if you are looking for a CRU that can meet Phase I manufacturing requirements.
Benefit #2 – Timeline Reduction
The second benefit of Phase I drug production at a CRU cGMP pharmacy is timeline reduction. A full run of GMP product at a contract manufacturing organization (CMO) takes significant time and investment plus a full supply of active pharmaceutical ingredient (API). Phase I manufacture, however, requires only a small batch of the compound, using far less API. A CRU cGMP pharmacy can supply a small run quickly, on-site, with a QMS and data to meet regulatory requirements.
You gain significant control over your schedule for Phase I studies with cGMP manufacture at the CRU versus at a CMO. After the API arrives at the CRU pharmacy and is released for use by GMP Quality Assurance (QA), the staff can manufacture doses quickly. Also, sponsors appreciate the reduction in stability testing and data needed when the CRU makes drugs on-site and administers them immediately. Not having to run full-fledged stability studies saves significant time.
Another timesaving involves the data you gain during the cGMP manufacturing process. Master Batch Records detail the drug manufacturing process, and accountability logs document drug administration. The information is available immediately on-site. Evaluations of the final drug product are provided as part of the GMP dose analysis, detailing characteristics such as ID Test, Purity and Strength provided on a Certificate of Analysis. The benefit? You have the criteria you need to ensure quality.
最后,赞助商享有在现场药物制造中的用量“在飞行”调整的灵活性和速度。药房是在小批处理中生产胶囊或口服溶液,现场制造允许您根据满足您的自适应试验设计需求的安全标记调整协议设计并制定最后一分钟的剂量调整。
Benefit #3 – Cost Efficiency
I阶段在CRU制造的第三个好处需要提高成本效率和降低财务风险。成本是执行临床试验的主要因素。您可以通过开始一个完整的CMC活动来降低您的财务风险,只能根据晚期毒理学结果或安全数据取消或更改配方。CRU的制造剂量允许您适应配方中的可变性,而不会产生昂贵的延迟。
随着CMO的大型药物制造,如果您需要更改配方,您将浪费大量API。请记住,合同开发和制造组织(CDMO)需要大量批量,用于创建药品的最低尺寸;然而,I阶段试验不需要大量的药物,因此很少有API的小型运行可以容易地满足您的需求。
We estimate that cGMP at a CMO results in 200-300% excess API, while cGMP at a CRU results in only 1-25% excess API (see Table 1).
通过在I阶段制造,您可以做出明智的决策并有效地使用您的材料。您还可以通过增加本地控制,因为您有能力在单个空间内维护您的药物而不是在多个位置工作。您可以控制您的配方和时间表,维护质量,提升API管理,以获得更高质量的过程。
Manufacturing your Phase I drug at the CRU versus a CMO is the more progressive process and part of the advantage to an integrative relationship between sponsor and CRU. A cGMP pharmacy at the CRU clearly provides a lower-cost alternative for Phase I drug manufacture.
福利综述
该表是比较I阶段药物制造的三个不同空间的摘要──根据质量,时间,数量,成本和风险。您可以在CRU上看到CGMP提供高质量,更短的时间,更短的API浪费,与替代方案相比,您的程序的降低成本和中性风险。
表1:I阶段药物制造来源的比较效益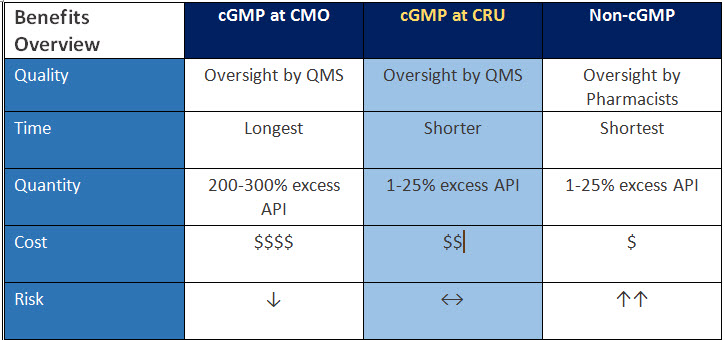
Exploring Benefits by Study Type
Next, let’s explore benefits of Phase I cGMP drug manufacture at the CRU according to study type:
- Exploratory IND trials – provides small amount of doses for subjects in Phase I studies
- 第一次人类研究 - 提供在试验网站制造剂量的灵活性,以帮助管理成本和时间表
- Absorption, metabolism and excretion (AME) studies – meets quality demands with speed and allows exploration of micro-dosing for Phase 0
- 单一升序剂量(悲伤)/多个升序剂量(MAD)试验 - 适用于适用于给药的现场变化,以获得最大的灵活性和时间
- 需要快速剂量变化的试验─使用CGMP工艺保持质量
Streamlining the Process
制造阶段我在CRU流药物lines your process from beginning to end, providing quality and flexibility to meet your needs. For example, these types of studies present special challenges and present opportunities for benefit by cGMP at the CRU:
- AME – handling hot/cold radiolabeled material quickly on-site for proper evaluation
- 悲伤/疯狂的剂量,提供药学上优雅的配方,导致II期和商业方法
您可以看到CGMP CRU药房独特地定位以适应早期临床开发中的自适应试验设计。
啮合制造和试验
Integrating cGMP manufacturing and clinical conduct at the same facility allows more control over the process, and therefore over timelines and costs. Meshing the processes requires the construction of clean rooms (ISO 7 and ISO 8) for sterile and non-sterile manufacturing in controlled environments. Additional working enclosures must meet ISO 5 air quality standards for sterile manufacturing or assembly (such as a BSC and CACI), and containment equipment includes powder cabinets for non-sterile manufacturing.
必须验证GMP空间以进行各种类型的药物制剂,包括:
- Capsules (liquid and powder)
- Oral solutions and suspensions
- 可注射(无菌)溶液
Updated standard operating procedures maximize efficiency and consistency. These include procedures for drug receipt, cleaning and gowning plus QA) release to the clinic. Manufacturing at the CRU also requires a robust environmental monitoring program plus final drug product sterility and endotoxin testing to meet USP <71> and <85> under cGMP conditions. The Phase I cGMP pharmacy must accommodate technical batches or engineering runs to deliver confidence to sponsors regarding their final drug product.
一个大益处:消除从CRU制造套件转移到诊所的产品,因为剂量直接向CRU护士提供,没有所需的运输或包装。
Sponsors of early clinical drug development are always looking for tools to help reduce timelines and manage costs. Why?
制造成本估计为整个发展的20-40%of a drug product through all phases.1
在早期临床开发期间花费了大部分投资。如果我们通过根据CGMP指南在CRU现场提供阶段的审判药物,我们可以帮助降低成本和时间表,这是赞助商的主要福利。你能节省多少时间和金钱?
The Covance Approach
景can help you make informed decisions about your formulation to be compliant with FDA cGMP guidance for the design, monitoring and control of your drug manufacturing. Working together, we can achieve theThree Big BenefitsCGMP药物制造在CRU中,我的阶段试验:质量和安全,时间轴减少和成本效率。
- 赵Y,Fleischhacker A.在新药物的临床开发期间合同开发和制造成本。(2013)Applied Clinical Trials2013 Aug 05













