患有93克罗恩病(CD)和168阶段溃疡性结肠炎(UC)I-III行业赞助的研究计划和开放入学,重点关注研究炎症性肠病的新疗法(IBD)1。
Remission is the main aim of IBD therapy, but IBD studies often face challenges with minimizing the placebo effect2,3,4。安慰剂效应可以分类为安慰剂响应/益处(患者证明改善)或安慰剂缓解(患者实现缓解)。认为影响安慰剂效应水平的因素可能是矛盾的,具体取决于研究的重点是否在安慰剂响应/福利或安慰剂缓解时5.。
检查CD和UC研究中的安慰剂缓解率,简要介绍了Meta分析,揭示了一些有趣的模式。
表A:报告了关键因素对安慰剂缓解率在IBD研究中的影响
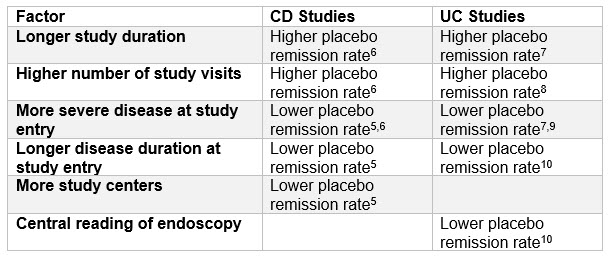
上面的发现与几个关键主题相处得很好:
- 设计患者为中心的研究– “Patient centricity” within studies has become a key priority in recent years. With so much competition, your study has to be more “attractive” than the others. Reducing the number of clinic visits and study duration is a simple means of doing this and is more likely to result in a lower placebo remission rate6,7,8.(尽管研究持续时间必须明显地考虑到研究药物的药效动力和监管指导,例如欧洲药物局(EMA)指导2,3).
- 竞争性试验环境对网站数量的影响- 在运行临床研究时,自然趋势是为了尝试利用最小数量的网站和财务原因来提供研究。然而,事实证明,竞争激烈的IBD试用环境可能会对我们迫使我们利用更多的网站来努力,否则我们可能会策划,这可能有助于降低安慰剂率5.。
- Patient Selection for Studies Targeting Moderate-Severe Disease- 期望在靶向患者持续时间的患者的研究中观察到较低安慰剂缓解率的发现是合理的5.,10and/or have a greater disease severity5,6,7,9.(那些患病严重程度的人通常具有较长的持续时间)。这些患者的可能性不太可能自发地进入疾病缓解。然而,这对这些研究患者患者通常具有较小的疾病严重程度的患者来说并不好消息。因此,在这些研究中,甚至更重要的是引入其他举措,以最大限度地减少安慰剂率(见下文)。
- 好科学- 鉴于客观的研究端点长期以来,已知提供更强大的数据,EMA对CD的指导方针2和uc.3.studies now propose endoscopic remission as a co-primary endpoint in efficacy studies (and centralized reading of endoscopies is highly encouraged). The requirement for endoscopy to confirm remission is also supported within the U.S. FDA draft guideline for clinical trial endpoints in UC studies4.。The referred to meta-analyses would further tend to support this approach as there is evidence, certainly with UC, that centrally read endoscopies lead to lower placebo remission rates10.。
There are other initiatives that sponsors can and should employ with the aim of minimizing placebo rates, such as:
- 在资格标准中制定没有歧义的协议
- 公开地向现场人员发言,了解安慰剂响应的潜力
- Declaring a state of enhanced surveillance by examining quantitative patterns of the study, such as screen failure rates
- 鼓励现场人员创造一个研究联盟,而不是现场和患者之间的通常治疗联盟
Finally, sponsors need to ensure patients fully understand that the purpose of the study is to determine if the drug is effective while personnel involved in the study should be trained to avoid raising patients’ expectations that the medication will be effective. These joint efforts, along with patient-centric practices, can help avoid inducing a high placebo response when studying new therapies for IBD.
Learn more about optimizing your clinical development outcomes with the right partner.
参考资料
- Citeline Trialtrove (January 2020)
- 新型药品开发治疗克罗恩病的指导方针。CPMP / EWP / 2284/99 Rev.2
- Guideline on the development of new medicinal products for the treatment of ulcerative colitis. CHMP/EWP/18463/2006 Rev.1
- 溃疡性结肠炎:工业临床试验终点指导(草案)。美国食品和药物管理局(FDA)
- Jaraith V等人。具有Meta分析的系统审查:Crohn病的诱导和维持试验中的安慰剂率。Aliment Pharmacol Ther2017; 45:1021-1042
- Chinyu Su等人。活跃克罗恩病临床试验中安慰剂缓解率的荟萃分析。Gastroenterology2004;126:1257-1269
- Jaraith V等人。Placebo response and remission rates in randomised trials of induction and maintenance therapy for ulcerative colitis (Review).Cochrane系统评价数据库2017, Issue 9
- inyckyj a等。溃疡性结肠炎安慰剂反应的定量。Gastroenterology1997年;112:1854-1858
- Jaraith V等人。P236 Placebo response and remission rates in ulcerative colitis clinical trials: systematic review and meta-analysis. ECCO Poster Presentations:Clinical: Diagnosis and Outcome(2015)
- macaluso f s等人。影响安慰剂臂临床和内窥镜结果的因素在溃疡性结肠炎的生物学和小分子药物试验中的试验:Meta分析。Inflamm Bowel Dis: Volume 25, Number 6, June 2019











