Comprehensive dendritic cell analysis by flow cytometry using the new CompDC™ panel
AUTHOR:
David Draper, PhD | Associate Director, Scientific Development
DATE:
February 2019
T cell-mediated anti-tumor responses hinge on the activity of antigen-presenting cells (APC) that present tumor-associated antigen (TAA) and provide co-stimulatory signals to T cells. This in turn activates tumor-specific T cells, triggering their expansion and recruitment to the tumor where they exert their effects. Dendritic cells (DC) are professional APCs that are instrumental in this process. DCs can be activated to express numerous costimulatory ligands and can internalize antigen and present immunodominant peptides to both CD4+and CD8+T cells. The development of novel therapeutics that harness DC function is an area of intensive investigation. To facilitate these efforts and address the growing need for robust comprehensive DC immunophenotyping in murine pre-clinical models, Covance has configured a new standard panel, CompDC™. In this Tech Spotlight, we will demonstrate how various DC subsets in tumor and other tissue-derived cell samples can be analyzed using this panel.
For a general introduction to the different DC subsets and their function in cancer biology, read our recent blog“An Introduction to Dendritic Cell Biology and Analysis Using Syngeneic Immuno-Oncology Models”
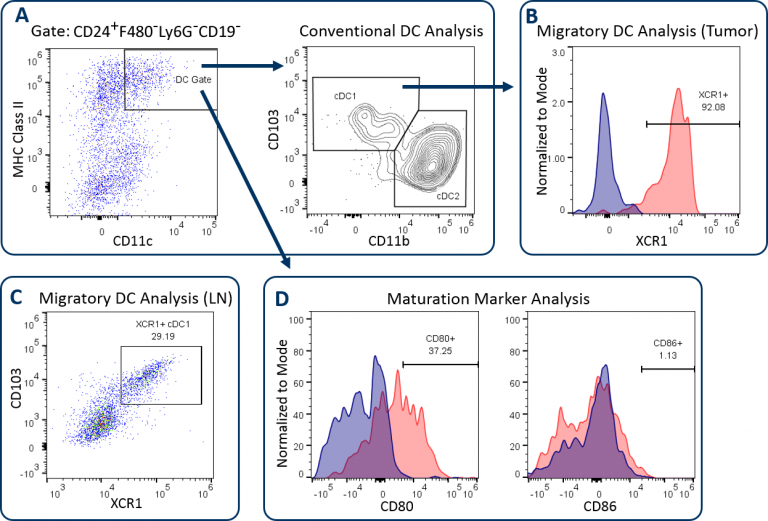
The CompDC™ panel allows investigators to determine ifin vivotherapy affects the abundance and activation state of different DC subsets in the tumor, tumor-draining lymph nodes (LN), or other host compartments. It is a 12-color panel that combines a set of antibodies that when properly examined, delineates distinct DC subsets, and provides insight into the T cell activation potential of the DCs (Table 1). Through a meticulous gating strategy, the panel first facilitates DC analysis with precision by excluding macrophages, granulocytes, and B cells using an exclusion gate. This process minimizes the risk that DC analysis is compromised due to contamination by irrelevant or autofluorescent cells. CD24 expression is then used help delineate conventional DC subsets, which are generally classified by medium to high expression levels of CD11c and MHC class II. These conventional DCs include the CD103+/CD8+DC1 subset and the CD11b+DC2 subset (Figure 1A), which have been demonstrated to drive CD8+and CD4+T cell anti-tumor responses respectively.[1]XCR1 expression has been documented by multiple groups as a marker for DCs with cross-presenting activity, a phenotype required for DC-mediated activation of CD8+T cells.[2]Figures 1B and 1C demonstrate XCR1+直流分析B16-F10 tumor-derived cells and draining lymph nodes. XCR1 expression is generally associated with the DC1 subset, as shown. Finally, the expression of maturation markers CD80 and CD86 is measured to assess the T cell co-stimulatory potential of the DCs. Figure 1D shows that the expression of these markers is relatively low on DCs in B16-F10 melanoma tumors, indicative of the immunosuppression imparted on the DCs by the tumor microenvironment (TME).
Table 1: CompDC™ Panel Antibodies and Description of Their Utility
| Antibody/Dye | Description |
|---|---|
| CD45 | Pan immune cell marker |
F4/80, Ly-6G, CD19 |
Exclusion gate for macrophages, granulocytes, and B cells |
| CD11c | Dendritic cell marker |
| CD24 | Dendritic cell marker |
| CD8 | DC1 marker |
| MHC Class II | Dendritic cell marker/maturation marker |
| CD103 | DC1 marker |
| CD11b | DC2 marker |
| XCR1 | Cross presenting/migratory dendritic cell marker |
| CD80 | Maturation marker |
| CD86 | Maturation marker |
| Viability Dye | Dead cell exclusion |
| The CompDC™ can be customized to analyze inflammatory DC (InfDC) and plasmacytoid DC subsets by substituting in Ly-6C/CD206 and Siglec-H antibodies respectively. | |
The CompDC™ can also be customized for analysis of two additional DC subsets. This includes inflammatory DCs (infDCs), which is performed by substituting in anti-CD206 and anti-Ly-6C antibodies. InfDCs have been demonstrated to promote anti-tumor activity and can be delineated out of the monocytic myeloid-derived suppressor cell (M-MDSC) gate as CD11c+MHCII+CD11b+CD206+cells (Figure 2A).[3]Plasmacytoid DCs (pDCs) can also be quantified by substituting in an anti-Siglec-H antibody. While pDC function in the TME has not been fully characterized, many reports have demonstrated that these pDCs can have impaired function and can even have an immunosuppressive effect on anti-tumor responses.[4]pDCs generally have low levels of CD11c expression and are positive for Siglec-H, as shown in Figure 2B.

To learn more about the CompDC™ panel and how to incorporate it into your research,contact the scientists at Covance.
1Gardner, A., & Ruffell, B. (2016). Dendritic cells and cancer immunity.Trends in immunology,37(12), 855-865.
2Wylie, B., Seppanen, E., Xiao, K., Zemek, R., Zanker, D., Prato, S., … & Waithman, J. (2015). Cross-presentation of cutaneous melanoma antigen by migratory XCR1+ CD103− and XCR1+ CD103+ dendritic cells.Oncoimmunology,4(8), e1019198.
3Kuhn, S., Yang, J., & Ronchese, F. (2015). Monocyte-derived dendritic cells are essential for CD8+ T cell activation and antitumor responses after local immunotherapy.Frontiers in immunology,6, 584.
4Mitchell, D., Chintala, S., & Dey, M. (2018). Plasmacytoid dendritic cell in immunity and cancer.Journal of neuroimmunology.
Note: Studies were performed in accordance with applicable animal welfare regulations in an AAALAC-accredited facility
Comprehensive Dendritic Cell Analysis by Flow Cytometry Using the New CompDC™ Panel (PDF Version)
- Pan02-Luc: An orthotopic model of pancreatic cancer
- Bioluminescence imaging: In vivo monitoring of tumor development, disease dissemination and treatment efficacy
- 5TGM1-luc – A syngeneic murine model for multiple myeloma
- Functional Analysis of Tumor Infiltrating Lymphocytes by Flow Cytometry Using the Cytokine/Cytotoxicity™ Panel