Modeling liver cancer with syngeneic hepa 1-6: an update
AUTHOR:
谢Barnes博士|导演,三英洁具tific Development
DATE:
November 2019
Hepatocellular carcinoma (HCC) affects over one million people globally and is currently the third leading cause of cancer related deaths in the world.[1]Worldwide, there are 800,000 new diagnoses and 700,000 patient deaths each year. The incidence of HCC is highest in Southeast Asia and Sub-Saharan Africa due to high prevalence of hepatitis B and C viruses which strongly predisposes one to develop chronic liver disease and subsequent HCC.[1,2]In the United States, there are expected to be 42,030 new diagnoses and 31,780 patient deaths in 2019.[2]Depending on the stage of disease, HCC can be treated with surgery, chemotherapy, targeted therapies, and radiation.[4]Current clinical trials are tackling unresectable or advanced stage HCC with mono or combination immunotherapy, monoclonal antibodies or oncolytic virus therapy.[1-4]These approaches have demonstrated tumor shrinkage and improved survival, but are not curative. Hence, there is a need for improved treatment options. Covance has developed the syngeneic HCC model, Hepa 1-6, for preclinical evaluation of immunomodulatory drug candidates.
Hepa 1-6 is a murine hepatoma derived from the BW7756 hepatoma that arose spontaneously in C57L/J mice, which contrasts with most available hepatoma models (BNL, A.7R.1, MH-129, MH134 and MH-22A) that are chemically transformed or induced lines. Therefore, the Hepa 1-6 tumor model established in immunocompetent mice represents a clinically relevant model for preclinical testing of immunotherapy.
审查早期数据
In our2018 Hepa 1-6 Model Spotlight, we presented initial subcutaneous growth of the model and response data following dosing initiation with small but established tumors (mean tumor volume ~80-90 mm3). Treatment of Hepa 1-6 with checkpoint inhibitorsin vivo对早期疾病非常有效,导致50-100%的耐用响应,但与毒品候选人结合留下了一小余地。在2019年,我们继续努力改进该模型,以使检查点抑制作为具有调查药物候选人的合理组合策略。在此,我们在更先进的皮下皮下HEPA疾病模型中呈现抗肿瘤反应数据。
Checkpoint Blockade Response Data for Hepa 1-6
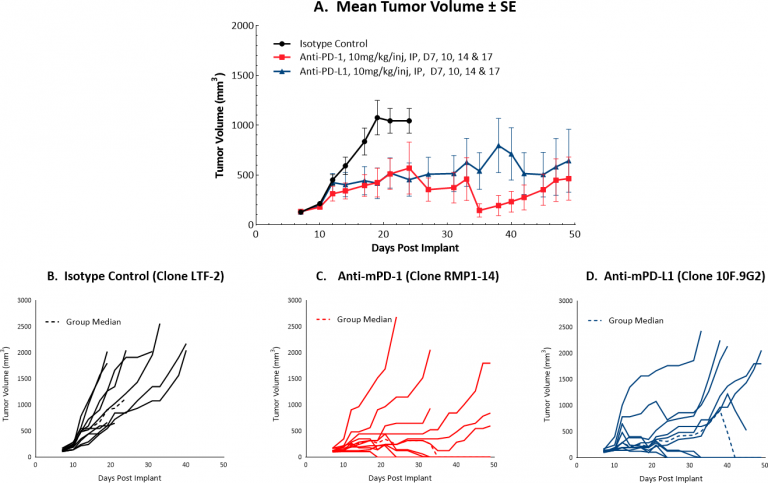
Initiating treatment with more advanced Hepa 1-6 tumors (at a mean tumor volume of ~130 mm3) resulted in a favorable response for testing drug candidates in combination with checkpoint blockade. Figure 1 illustrates mean (A) and individual (B-D) growth of control tumors and those treated with anti-mPD-1 or anti-mPD-L1 monotherapy. At this increased starting tumor volume, each monotherapy produced 40% durable complete responses, suggesting that while this strategy can certainly offer a means by which to test rational combination strategies, it is possible that the model can be further optimized. We will continue to refine this model to allow checkpoint blockade to maintain a moderate response, ideally with fewer complete responses. Small differences in mean tumor volume may mean large differences in response, so we are systematically approaching this challenge stepwise, with an ongoing study testing anti-tumor efficacy of checkpoint blockade with dosing initiation at a mean tumor volume of ~150 mm3。本研究尚未完成,但与较低的启动肿瘤卷(数据未显示)相比,已经表明较少的完全响应。
为了进一步增加该HCC模型的效用,我们有荧光素酶 - 使能HEPA 1-6细胞系(HEPA 1-6-LUC)。目前正在优化该线以用于肝癌的原位建模。荧光素酶标签将用于通过直接进入肝脏后通过生物发光成像(BLI)监测肿瘤进展。
Advisement for Using the Hepa 1-6 Model in Your Next Liver Study
The Hepa 1-6 model provides robust preclinical means by which to study hepatocellular carcinoma. Further, the strong response to checkpoint inhibition coupled with flow cytometry analysis is suggestive of a minimally immunosuppressive tumor micro-environment, therefore, the Hepa 1-6 model can be considered immunologically “warm.” This immunologically favorable phenotype is highly attractive for those developing therapeutic agents posited to stimulate the immune system.
Please联系人to speak with our scientists about how Hepa 1-6 or one of our othersyngeneic modelscan be used for your next immuno-oncology study.
1Medavaram, S and Zhang Y, 2018. Emerging therapies in advanced hepatocellular carcinoma. Exp Hematol Oncol 7:17.
2https://www.cancer.net/cancer-types/liver-cancer/statistics.
3Pinter M & Peck-Radosvljevic M. 2018. Review article: systemic treatment of hepatocellular carcinoma. Aliment Pharmacol Ther. Sep; 48(6): 598–609.
4Waidmann O. 2018. Recent developments with immunotherapy for hepatocellular carcinoma. Expert Opin Biol Ther. Aug;18(8):905-910.
注意:研究是根据AAALAC认可的设施中适用的动物福利法规进行的